Analytical pyrolysis is a powerful tool for the qualitative analysis of polymers. The analysis usually starts from a simple pyrogram match to an existing pyrolysis database to identify the polymer chemical structure. If multiple polymers are identified, a quantitative method is often adopted by comparing peak areas of the pyrolysis products to determine each polymer ratio. In this application, styrene-isoprene block copolymers, which are large-volume, lowcost, commercial thermoplastic elastomers, are analyzed by following this approach.
Block copolymer standards styreneisoprene at 14, 17 and 22 percent (styrene to copolymer weight ratio) were obtained from Sigma Aldrich. Solutions of each copolymer standard were prepared in tetrahydrofuran to 1 mg/mL. A 5μL aliquot of each weight percent copolymer solution was added to a Drop-In-Sample Chamber (DISC) tube, then pyrolyzed to a setpoint of 600°C using a CDS 6150 Pyroprobe.
Pyoprobe
Setpoint: 600°C 30 seconds
DISC Interface: 300°C
Transfer Line: 300°C
Valve Oven: 300°C
GC-MS
Column: 5 percent phenyl (30m x 0.25mm)
Carrier: Helium 1.25mL/min, 75:1 split
Oven: 40°C for 2 minutes
10°C/min to 300°C
Ion Source: 230°C
Mass Range: 35-600amu
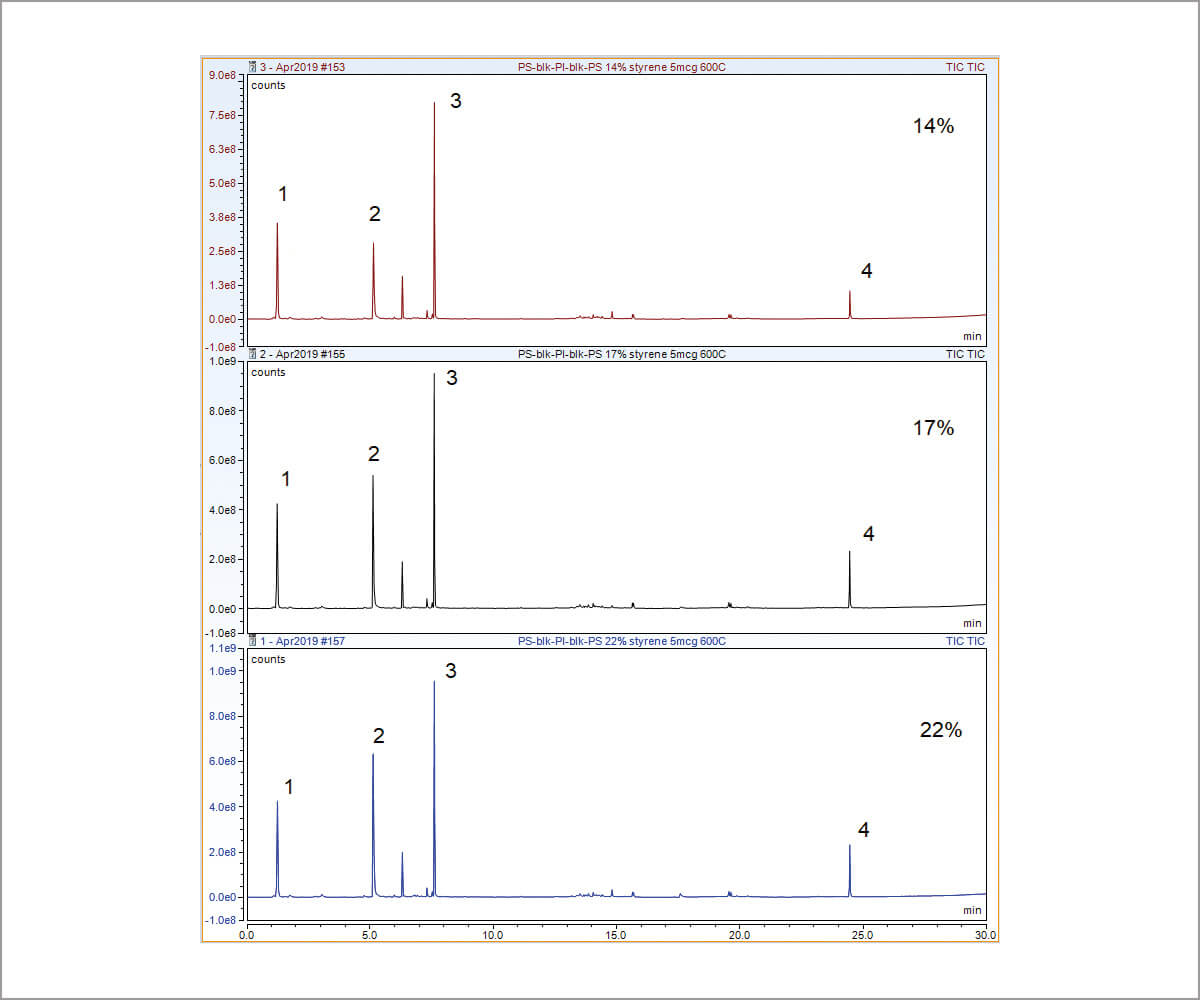
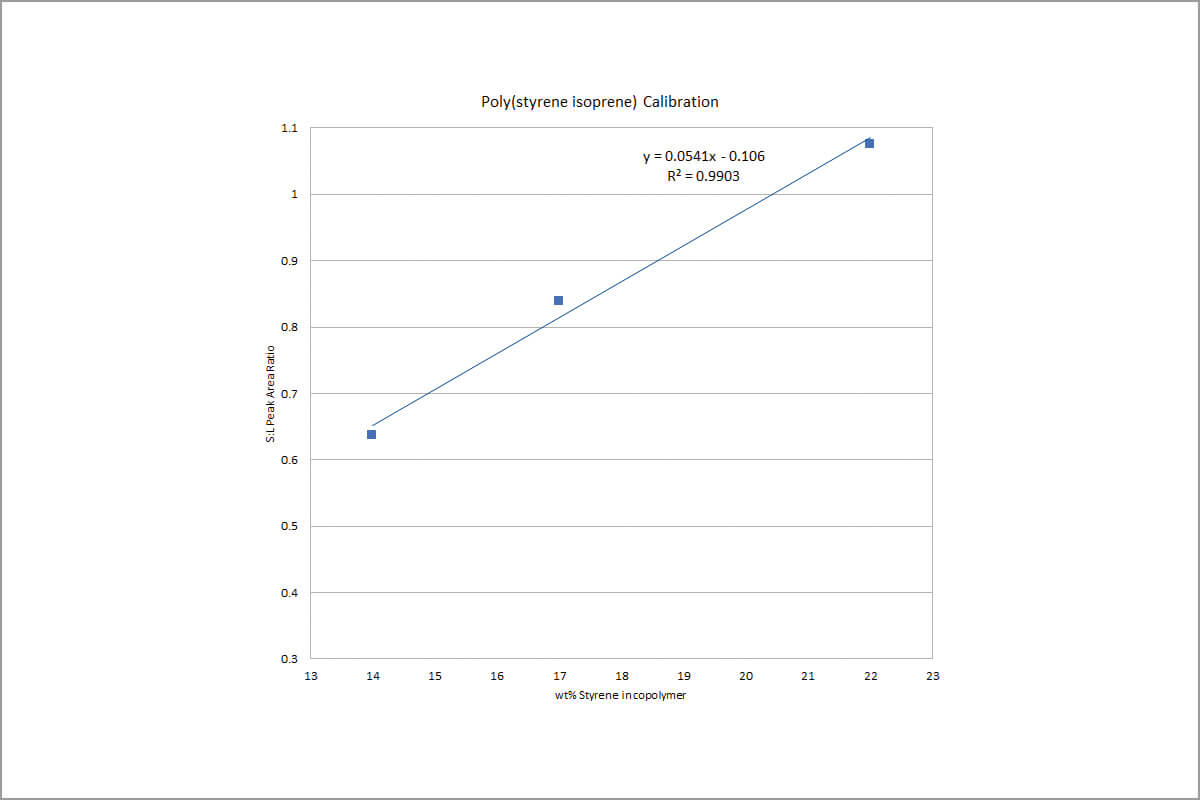
Figure 1 shows pyrograms of poly(styreneisoprene) copolymers containing 14, 17, and 22 weight percent styrene.. When pyrolyzed, polystyrene is principally broken down to monomer (Peak 2 in Figure 1) and trimer (Peak 4 in Figure 1). As the styrene weight increases in the copolymer, so does the area of the peaks from polystyrene (Peaks 2 and 4) in relation to the peaks from polyisoprene (Peaks 1 and 3).
Considering the signal to noise ratio and the simplicity of algorithm, the highest peaks from styrene monomer (Peak 2) and isoprene dimer (Peak 3) were chosen for quantitative analysis. Area ratios of these two peaks were plotted against the weight percent of styrene in each of the standards in Figure 2, which shows a linear calibration with an R2>0.99. The reproducibility study was also carried out from seven sample runs on the 17 percent styrene standard. An RSD of 1.13 percent is obtained in Table 1.
The linearity and RSDs demonstrate that the latest version of the Pyroprobe from CDS is adept at the quantitative analysis of copolymers.






