Introduction
Interest in chromatography using hydrophilic interaction liquid chromatography (HILIC) has continued to build in recent years. Full adoption of the technique, however, has been slowed by experiences of poor reproducibility. Reproducibility issues generally stem from a lack of understanding of the controls one sets up in a chromatographic system. Re-equilibration times in HILIC, for example, have been reported as being exceptionally long as compared to reversed-phase chromatography. Is it possible, then, that some reproducibility issues are a result of improper re-equilibration settings?
In this study, re-equilibration times in HILIC, for both aqueous:organic gradients and buffer gradients are systematically explored. Repeatability of retention and selectivity was studied as a function of equilibration times following aqueous:organic gradients.
The impact of the use of buffer gradients and subsequent equilibration procedures on retention times were also investigated using several HILIC stationary phases and probes. The results not only promise to improve method development practices, but also provide valuable insight into HILIC retention mechanisms across a diverse set of polar stationary phases.

Experimental
Organic: Aqueous Gradient Study Using a bare silica (Ascentis Express HILIC, 10 cm x 3.0 mm, 2.7 μm) and a pentahydroxy phase (Ascentis Express OH5, 10 cm x 3.0 mm, 2.7 μm), a set of neutral molecules was run employing a gradient from 5% aqueous to 50% aqueous with varied equilibration times. The same study was also performed using a gradient from 5% to 25% of the aqueous component. Mobile phase A was 5 mM ammonium acetate in 95% acetonitrile while mobile phase B was 5 mM ammonium acetate in 50% acetonitrile. Mobile phase C was 5 mM ammonium acetate in 75% acetonitrile. Gradient 1 ran from A to B in 10 minutes. Gradient 2 ran from A to C in 10 minutes. Re-equilibration times were set at 2, 5, 10, 15 and 20 minutes. The column temperature was held at 35 ºC, flow rate at 0.5 mL/min, injection Volume at 2 μL and detection by UV absorbance at 254 nm. Initially, a set of neutral polar analytes (adenosine and cystosine) were injected in triplicate. To represent polar basic molecules, metanephrine and normetanephrine were also tested.
Buffer Gradient Study
The buffer gradient study was run identically to the organic:aqueous study except that mobile phase A consisted of 2 mM ammonium acetate in 90% acetonitrile, pH* adjusted to 7.0 (+/- 0.05) with acetic acid and mobile phase B consisted of 10 mM ammonium acetate in 90% acetonitrile, pH* adjusted to 7.0 (+/- 0.05) with acetic acid (pH measured in the presence of organic and calibrated using aqueous standards). The gradient was run from 100% A to 100% B in 10 minutes. In addition to the polar neutral mix and the polar basic mix, an additional nonpolar basic mix was included.
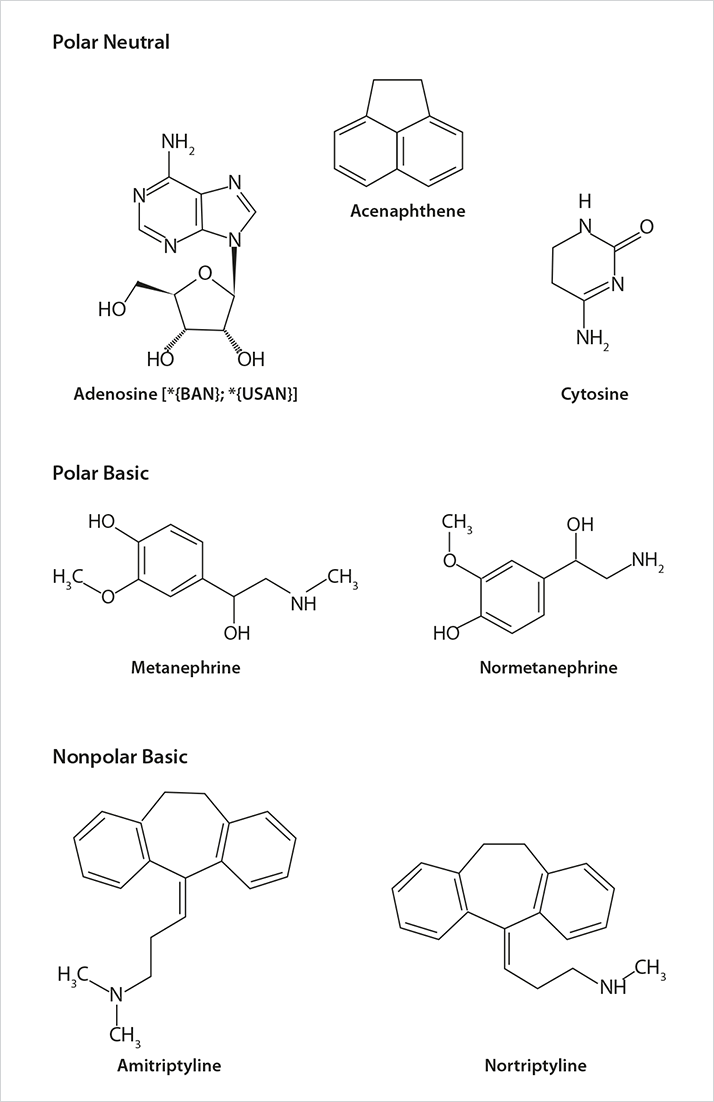 Figure 1. Structures of the Study Probe Molecules
Figure 1. Structures of the Study Probe MoleculesResults and Discussion
Figure 1 shows the structures of the test probes used throughout the study. Adenosine and cytosine represent neutral polar molecules that should only interact with the stationary phase via partition or polar (dipole type) interactions. The metanephrines are both polar and positively charged under the conditions of the study and thus can interact via partitioning, polar and ionic mechanisms. Lastly, the tricyclic antidepressants (TCAs) amitriptyline and nortriptyline are ionized, but relatively nonpolar. The TCAs, then, are expected to retain via ion-exchange mechanisms and perhaps some polar interactions, but not via partition.





