Introduction
A new high efficiency GFC column, Yarra, was recently introduced and is significantly more efficient than other GFC columns on the market. In addition to higher efficiency, Yarra columns demonstrate significantly higher inertness to ionic interactions versus other GFC columns; however, such chemical characteristics sometimes require changes to operating parameters. Performing method development for protein aggregation analysis using next-generation Yarra GFC columns will be discussed.
 TN-1131 APPLICATIONS
TN-1131 APPLICATIONSBeing an isocratic method, gel filtration chromatography is assumed to be a simple method with little or no method development involved. On closer inspection, however, subtle changes in mobile phase and other parameters can have significant results on separation performance and accuracy of determining the aggregation state of a protein. Protein gel filtration columns are typically made by bonding a highly polar “diol-like” ligand to a porous silica matrix of a specific pore size. This polar “diol” coat on the silica is intended to minimize surface interactions between the silica and proteins, resulting in separations based on the size of a protein in solution as proteins are differentially excluded from the pores of the silica particle. One typically uses different pore-sized columns that provide maximum resolution of a specific molecular weight range based on the protein being separated. Often, two different pore-sized columns overlap in a molecular weight range, resulting in different selectivities based on the column being used. Sometimes a column at one edge of the overlap demonstrates better resolution in a protein specific manner. Since protein separations are typically looking at trying to quantitate non-covalent aggregates in solution, it is most common that a buffered aqueous mobile phase is used for GFC separations. While coating GFC silica with a polar bonded phase greatly reduces the amount secondary interactions between proteins and the silica matrix, the reality is that some secondary interaction persists. The secondary interactions can be summed up into two categories: ionic interactions between acidic free silanol groups on the silica surface and basic residues of a protein, and hydrophobic interactions between the bonded phase ligand and hydrophobic pockets of a protein. Especially aggregated proteins tend to be more sensitive to hydrophobic and ionic interactions. Depending on the protein and column being used, different running conditions can be utilized to minimize such secondary interaction, providing for more accurate quantitation of low level impurities in a protein product. Examples will show how mobile phase composition and column selection can have a major impact on separations and factors to consider in developing a gel filtration method using the new Yarra brand of GFC columns.
Material and Methods
Standard proteins and mobile phase modifiers were purchased from Sigma Chemicals (St. Louis, MO, USA). Additional test proteins were purchased from R+D systems (Minneapolis, MN, USA). Mobile phases were obtained from EMD (San Diego, CA, USA). The instrument used for all separations was an Agilent® 1200 HPLC with an autosampler, column oven, and multi-wavelength detector. All separations used Yarra SEC-2000 and Yarra SEC- 3000 columns (300 x 7.8 mm) obtained from Phenomenex (Torrance, CA, USA). Mobile phases with varying molarities of phosphate and sodium chloride were used (noted in each chromatogram).
Results and Discussion
Column Selection
Gel filtration chromatography separates proteins based on their size in solution and how much a particular protein can permeate into the pore of the bonded silica particle used in the column packing. Larger proteins will permeate a decreased distance into the porous particle versus smaller proteins which will permeate deeper into the particle. As a result, fully excluded aggregates and large proteins will elute first, followed by multimers, then the fulllength monomer protein, and then protein fragments with salts and small molecules coming out last. Each specific Yarra column has an optimal molecular weight separation range which is based on the pore size of the porous media: Yarra SEC-4000 (500 Å), Yarra SEC-3000 (290 Å) and Yarra SEC-2000 (145 Å). Figure 1 lists the suggested molecular weight range of each media based on the mobile phase conditions used. Note that there is significant molecular weight overlap between each individual phase. Depending on the separation goal of a specific GFC application, one or the other phase may be appropriate.
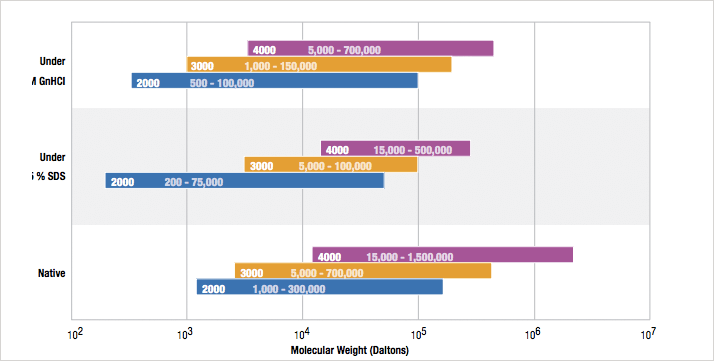 Figure 1. Table of suggested separation ranges for particular Yarra SEC columns based on a specific class of mobile phase used for the separation (native or denaturing). Note that the three phases (SEC-2000, SEC-3000, and SEC-4000) all overlap under all conditions; in many cases more than one phase might be appropriate for a separation.
Figure 1. Table of suggested separation ranges for particular Yarra SEC columns based on a specific class of mobile phase used for the separation (native or denaturing). Note that the three phases (SEC-2000, SEC-3000, and SEC-4000) all overlap under all conditions; in many cases more than one phase might be appropriate for a separation.While the focus is more on buffer influences, comparing Figure 2 and Figure 3 demonstrates that in overlap region, application goals may influence column choice. The separation between Ig-G dimer and monomer (Rs 1,2) is slightly better on the Yarra SEC- 3000 while resolution between Ig-G monomer and BSA (Rs 2,3) is slightly better on the Yarra SEC-2000. For any overlapping molecular weight region, both columns should be evaluated for that application.





