By Karen Sam
Certain phthalate additives are known to be harmful to humans, resulting in regulations regarding their use. With growing environmental awareness and perceptions, the use of phthalates has been restricted in many countries, including the European Union and the United States of America. As a result, a few international standards and conformity assessment bodies such as the International Electrotechnical Commission (IEC) and the American Society for Testing and Materials (ASTM), have published standards for determining certain phthalates in polymeric materials. The recent IEC Method 62321-8 defines approaches to determine di-isobutyl phthalate (DIBP), di-n-butyl phthalate (DBP), benzyl butyl phthalate (BBP), bis-2-ethyl hexyl phthalate (DEHP), di-n-octyl phthalate (DNOP), di-isononyl phthalate (DINP) and di-iso-decyl phthalate (DIDP) in electronics using GC-MS and TD-GC-MS.
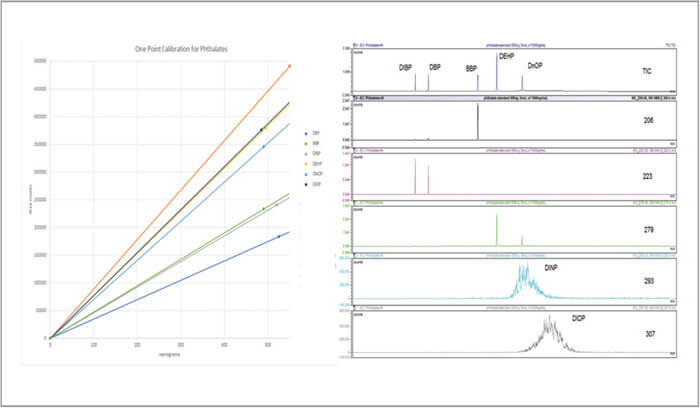
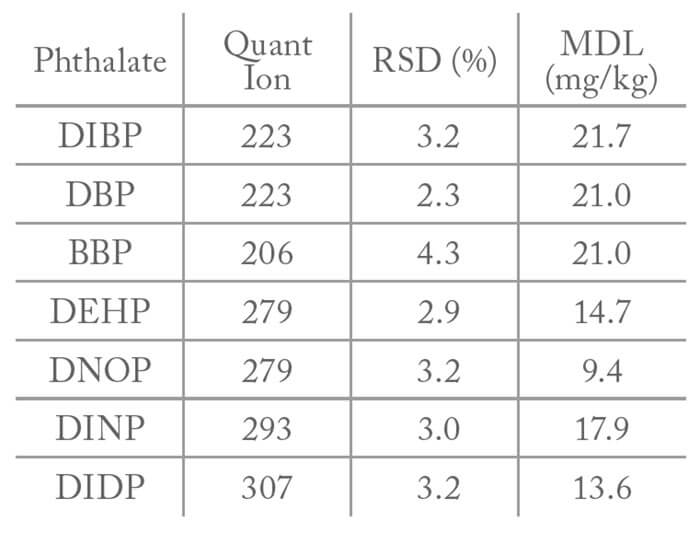
The statistical measures related to reproducibility depend on temperature precision, along with sample related issues like homogeneity and sample preparation. Eight replicates a 500ng phthalate standard provided area RSDs of around 3% for most of the phthalates, which gives a very small statistical variation. Furthermore, when method detection limits were studied in accordance with the IEC method, seven replicates produced calculated MDLs ranging from 9.4 to 21.7 mg/kg, 78-91 percent lower than the 100mg/kg requirement (Table 1).
The latest version of the Pyroprobe from CDS Analytical ensures repeatable, reliable results for thermal extraction of phthalates in accordance with standard methods, like IEC Method 6321-8 for determination of phthalates in electrotechnical products.
Experimental Parameters
The samples were thermally extracted in a CDS Pyroprobe 6150 with an Autosampler, equipped with Drop-In-Sample-Chamber (DISC) technology. A DISC tube was used as the sample vessel.
Pyro Chamber:
Ramp 1: 200°C to 300°C at 20°C/minute
Ramp 2: 300°C to 340°C at 5°C/minute
IsoZones: 300°C
GC-MS:
Column: 5% phenyl (30m x 0.25mm)
Carrier: Helium, 50:1 split
Injector: 320°C
Oven: 80°C for 13 minutes | 20°C/min to 300°C
Mass
Range: 50-1000amu





