The purpose of process development in pharma is to select and optimize a synthetic route to produce the active pharmaceutical ingredient (API) by the safest, cheapest, fastest, and cleanest (by green chemistry where possible) route, following both Good Laboratory Practice (GLP) and Quality by Design (QbD) principles. As with any synthetic process impurities are generated, and for API development it is mandated by regulatory authorities (e.g., Federal Drug Agency—FDA, European Medicines Agency—EMA, etc.) that impurities are tracked and identified above a certain threshold, while genotoxic and mutagenic impurities must be reported at any level (as stated in the ICH Q7 guideline1).
Route scouting data, in process development, is typically stored in electronic notebooks. Associated analytical information may be accessible as PDF images stored within an experiment record. Unfortunately, however, from the perspective of entity characterization and review, the analytical data is not dynamically linked with the individual stage(s) of the process route and is unsearchable and inaccessible.
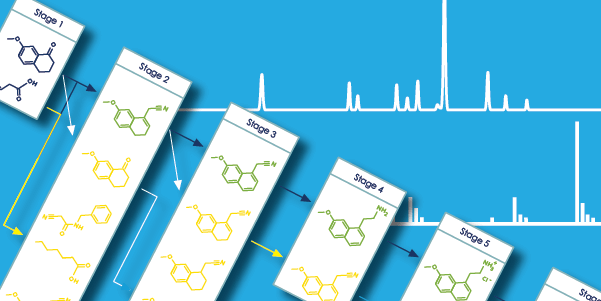
Effective management of API development data, and particularly impurity tracking, is necessary for the development of an optimal impurity control strategy. In order to successfully track the fate and purge of impurities, many scientists currently gather LC/MS and LC area percent values for impurity entities in MS Excel® spreadsheets, in lieu of chemically intelligent software to manage this data. While spreadsheets are effective for handling and managing numerical data—helping formulate and understand relationships through sorting and plotting—they are a weak tool for relating chemical structures with the analytical spectra and chromatograms used to identify and characterize them. For example, Excel is unable to generate an impurity map from the synthetic route or calculate purge factor from the available analytical data.
Here, we discuss Luminata™—software designed to help project teams assemble synthetic routes, associated impurities, and all relevant analytical data for process development in a systematized and searchable manner. Luminata enables effective inter- and intra-departmental collaboration and automatically calculates purge factors directly from LC/MS and LC data. In this document, we focus on two manual and often tedious workflows: process optimization and fate & purge calculations.