Abstract
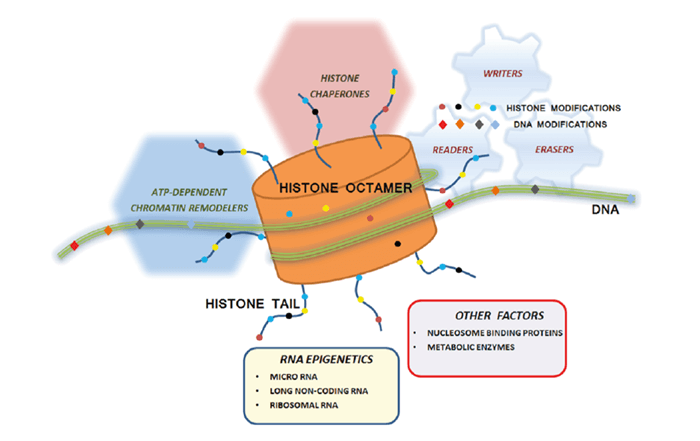
Epigenetic regulation of genomic DNA for gene expression is important in cellular differentiation and the development of an organism. Epigenetics also contributes to human diseases. This white paper summarizes how Isothermal Titration Calorimetry (ITC) is used for characterization of proteins involved in epigenetic regulation.

Introduction to epigenetics
Epigenetics is the study of heritable changes in gene expression caused by nongenetic mechanisms, without alterations in gene structure or DNA sequence. The epigenetic state of a cell evolves during the cellular differentiation and development of an organism, and epigenetic changes are linked to cellular reprogramming. Because epigenetic mechanisms may also be responsible for the integration of environmental responses at the cellular level, they potentially play an important role in the development of some diseases.