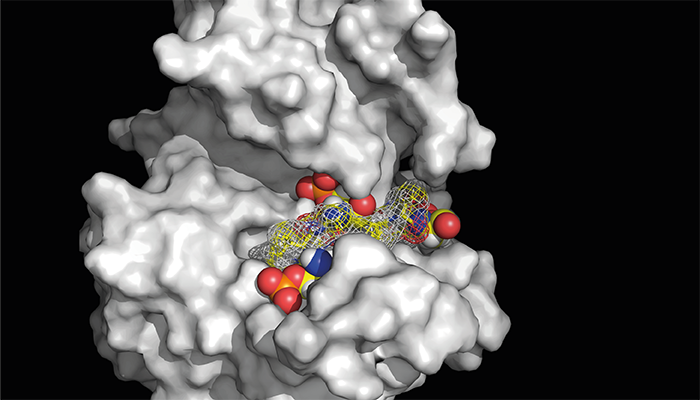
A peptide (shown in mesh) with attached phosphate tags (red and orange spheres) blocks the active site of CK1δ. Tagging the tail end of CK1δ, a process known as auto-phosphorylation, makes the protein less active, and with that less able to fine-tune the body’s internal clocks.
A set of molecular "tags" at the tail end of the casein kinase 1 delta (CK1δ) protein has been identified as a key regulator of the body’s circadian rhythms. By using nuclear magnetic resonance (NMR) and mass spectrometry techniques, scientists have pinpointed specific sites on CK1δ that control its activity, shedding light on how this protein governs the natural 24-hour cycles that influence sleep, metabolism, and other essential functions. The findings could open up new treatment pathways for circadian rhythm disorders and related health issues – or even lead to a treatment for jet lag.
In modifying other proteins, CK1δ acts as a crucial regulator of circadian rhythms. Though two isoforms of CK1δ, δ1 and δ2, were already known to exist, researchers had long been unclear on how small differences in their structures affected their functions.
“Our findings pinpoint three specific sites on CK1δ’s tail where phosphate groups can attach, and these sites are crucial for controlling the protein’s activity,” said Carrie Partch from the University of California, Santa Cruz, and co-corresponding author of the study, in a press release. “When these spots get tagged with a phosphate group, CK1δ becomes less active, which means it doesn’t influence our circadian rhythms as effectively. Using high-resolution analysis, we were able to pinpoint the exact sites involved – and that’s really exciting.”
The research team turned to NMR to understand how phosphorylation altered the molecular structure of CK1δ’s C-terminal tail and revealed that such modification causes the tail of the δ1 isoform to fold back onto itself, preventing it from efficiently interacting with other proteins that regulate circadian rhythms. In contrast, the δ2 isoform, which lacks the same sensitivity to phosphorylation, displayed less autoinhibition and remained more active.
The team then used HDX-MS to further probe the interactions between the phosphorylated sites and the main body of the protein. The combination of these techniques provided a comprehensive view of how phosphorylation regulates CK1δ activity, offering molecular-level insights that had previously eluded researchers.
“This technology allowed us to get to the bottom of a question that has gone unanswered for more than 25 years,” explained David Virshup, co-corresponding author from Duke-NUS, in the press release. “We found that δ1 interacts more extensively with the main part of the protein, leading to greater self-inhibition compared to δ2. When these sites are mutated or removed, δ1 becomes more active, which leads to changes in circadian rhythms.”
In addition to its role in regulating circadian rhythms, CK1δ is involved in other important processes, including cell division and neurodegenerative diseases. In other words, the team’s findings could have wider implications for developing treatments for conditions beyond sleep disorders, includingr cancers and neurological diseases.
“Regulating our internal clock goes beyond curing jet lag – it’s about improving sleep quality, metabolism, and overall health,” said Patrick Tan, Senior Vice-Dean for Research at Duke-NUS. “This important discovery could potentially open new doors for treatments that could transform how we manage these essential aspects of our daily lives.”
The next steps? Investigating how external factors, such as diet or stress, can influence these phosphorylation sites, further deepening our understanding of circadian control.




