I’m a PhD student at the University of Western Sydney (UWS) where I also obtained my undergraduate degree. My advisors are Marianne Gaborieau and Patrice Castignolles, and my research focuses on finding the relation between the branched structure of PAA (a pH responsive polymer) and its binding to and delivery of an anticancer drug (cisplatin). At the start of this year, I was awarded the Young Scientist Award lecture in the 7th International Symposium on the Separation and Characterization of Natural and Synthetic Macromolecules. I also received a student prize for International Polymer Meetings from the Royal Australian Chemical Institute, Polymer Division, for this symposium.
I have investigated a branched pH-responsive smart polymer, poly(sodium acrylate), PNaA, as a potential drug carrier for cisplatin. pH-responsive drug delivery systems are relevant for anticancer research as tumors have a more acidic environment than non-cancer cells. The presence of extensive branching is an ideal property for drug delivery applications as the branching provides better drug encapsulation. I have also developed a new method for monitoring the binding of anticancer drugs with charged polymers using capillary electrophoresis in critical conditions (CE-CC).
The aim is to bind an anticancer drug and a drug carrier together simply by mixing them in solution (without coupling agents) and to find a novel technique that monitors this binding. Drug delivery formulations usually involve a tedious synthetic process so inducing binding simply by mixing cisplatin and PNaA in solution and then leaving them to react is a much more straightforward approach. Moreover, promoting the use of CE-CC in terms of online monitoring of the binding and release of a drug to a drug delivery system is a novel idea that could be beneficial for other disease areas. As long as your samples are charged, I don’t see why you can’t use CE-CC – it’s cheap and doesn’t use much of your sample.
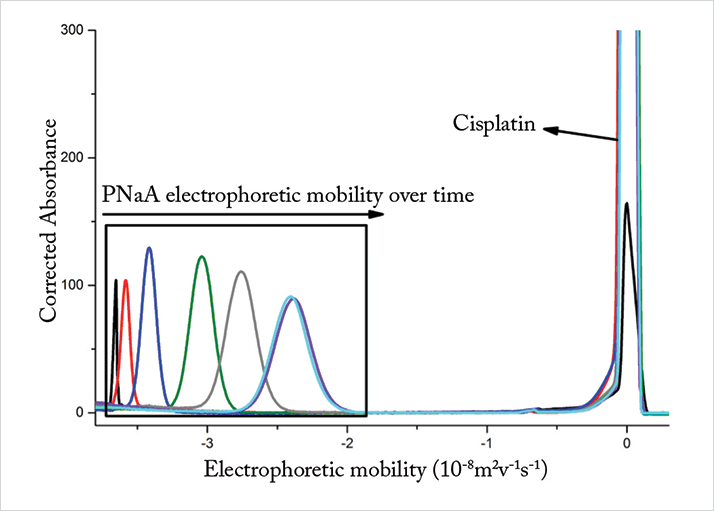
Online monitoring in CE-CC enables separation of the drug and PNaA during their binding. Free cisplatin, which is neutral, migrates the fastest followed by the negatively-charged PNaA (see Figure 1). The separations in this study are shown as a function of electrophoretic mobility, which depicts the intrinsic velocity of the species traveling in the capillary and corrects for the minor discrepancies the electroosmotic flow may cause from one injection to another. For our preliminary experiments, an increase in the electrophoretic mobility of linear PNaA was observed over time, suggesting that the polymer is binding to cisplatin (boxed region in Figure 1). Both the dissolution of cisplatin and the drug release from PNaA were also monitored using CE-CC. The drug release experiment was initiated once the binding was completed and a decrease in the PNaA’s electrophoretic mobility was observed. The activity of cisplatin bound to PNaA is investigated in vitro and other PNaAs with a higher degree of branching are also investigated.
Since this study, I have been optimizing the conditions for the release of cisplatin incorporated in PNaA. I have also done some preliminary in vitro testing with the PNaA/cisplatin formulation on a cancer cell line.




