When it comes to small molecules, synthetic chemistry is used to manufacture APIs. But in the world of biopharmaceuticals, biology dominates the process landscape. In this final part of our guide to biopharma, Andreas Mielcarek introduces us to the exciting topic of bioprocessing.
What exactly is bioprocessing?
Bioprocessing serves as a cornerstone in the manufacturing of monoclonal antibodies (mAbs) and other biopharmaceutical modalities, which are increasingly vital in therapeutic applications, particularly in oncology and autoimmune diseases. The process begins with the development of specific cell lines, typically mammalian, that are genetically engineered to produce the desired antibody. These cells are then cultured in bioreactors under tightly controlled conditions to maximize yield and maintain product consistency. Key steps in the downstream process include separation and purification, often achieved through techniques like chromatography and filtration. Throughout this process, stringent quality control measures are applied. These measures rely on advanced analytical methods, such as high-performance liquid chromatography (HPLC) to ensure the purity, structure, and function of the mAbs are preserved. This integration of biological sciences and process engineering not only enhances the efficacy of the antibodies but also ensures their safety and regulatory compliance.
How can the integration of online tools further enhance efficiency and quality control in bioprocessing?
Online LC as a process analytical technology (PAT) plays a crucial role in the real-time monitoring and control of bioprocessing, particularly in the production of mAbs. By integrating online LC systems directly into the bioprocessing workflow, manufacturers can continuously analyze the product stream for critical quality attributes, such as purity, aggregation levels, and glycosylation patterns. This real-time data allows for immediate adjustments in process parameters, ensuring that optimal conditions are maintained throughout the production cycle. The use of online LC as a PAT tool not only enhances the consistency and yield of the final product but also significantly reduces the risk of batch failures, leading to more efficient and cost-effective manufacturing processes. Furthermore, this technology supports regulatory compliance by providing comprehensive data for validation and quality assurance purposes, which is essential for meeting stringent industry standards.

(c) Agilent Technologies, Inc.
Reproduced with Permission, Courtesy of Agilent Technologies, Inc.
Could you explain the role and significance of bioprocessing in the biopharmaceutical industry?
Despite being hidden in the background – seemingly far removed from the drug names that make it into mainstream newspapers – bioprocessing sits at the core of the biopharmaceutical industry, busily and innovatively integrating advanced molecular biology, cutting-edge analytical techniques, and rigorous process engineering in the pursuit of optimized production of biotherapeutics. Indeed, bringing down costs while maintaining quality to boost accessibility is a modern driver of the bioprocessing field. Here, the use of sophisticated process analytical technologies, like online liquid chromatography, will be pivotal. By allowing real-time monitoring and precise control during manufacturing, we can benefit from enhanced efficiency while ensuring product quality and complying with strict regulatory standards.
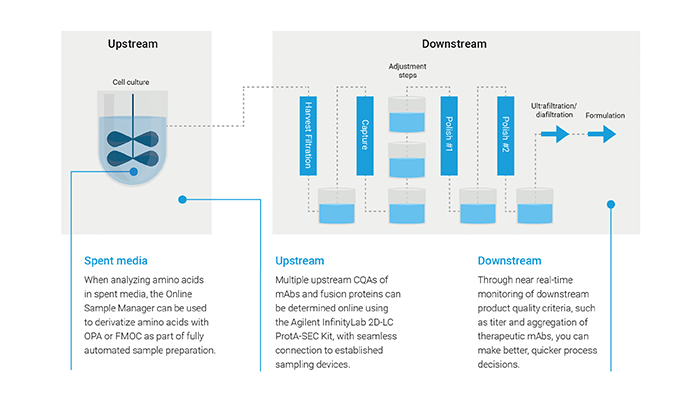
Figure 1. Biopharma process workflow.
© Agilent Technologies, Inc.
Reproduced with Permission, Courtesy of Agilent Technologies, Inc.
Bioprocessing is the backbone of the half-trillion-dollar biopharma industry – and analytical science plays a vital role at every step.
Agilent and the world of bioprocessing
What are the key components and benefits of Agilent’s solutions for online liquid chromatography monitoring in bioprocessing?
Agilent provides sophisticated solutions for Online LC with a specific focus on ease-of-use that can enhance efficiency and quality control in bioprocessing. Let’s explore five important areas to consider.
1. Real-Time Process Monitoring. Agilent’s Infinity II Online LC Solutions allows for the continuous and real-time monitoring of bioprocessing streams. This capability is crucial in biopharmaceutical manufacturing, where maintaining the consistency and purity of biologics like mAbs is essential for therapeutic effectiveness and safety.
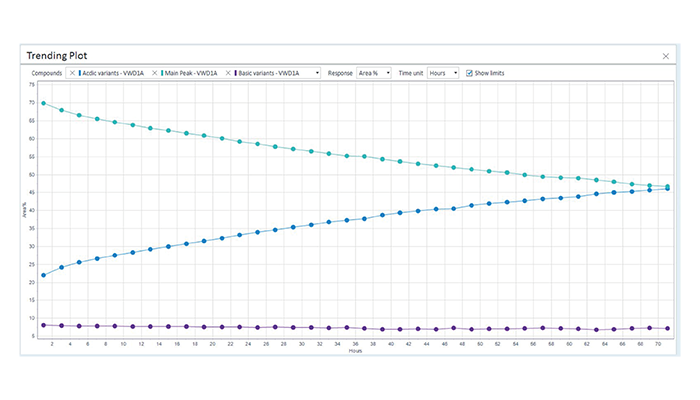
Figure 2. Visualization of a forced deamidation of a monoclonal antibody using an Agilent 1290 Infinity II Bio Online LC andAgilent Online LC Monitoring Software. The trending plot shows the decrease of the main peak (green), the slight decrease of basicvariants (purple), and the increase of the acidic variants (blue).
© Agilent Technologies, Inc.
Reproduced with Permission, Courtesy of Agilent Technologies, Inc.
2. Advanced Analytical Capabilities. The system employs high-resolution chromatographic techniques and detectors to analyze the critical quality attributes (CQAs) of mAbs. This includes assessments of purity, molecular size, and aggregation levels, which are key indicators of the quality of biopharmaceutical products.
3. Integration with Bioprocessing Equipment. The Online LC system is designed to integrate seamlessly with existing bioprocessing setups, including chromatographic purification systems. This integration facilitates inline sampling and analysis, thereby reducing the risk of contamination and sample degradation that can occur with manual handling.
4. Software for Enhanced Control and Compliance. The Agilent Online LC Monitoring Software plays a crucial role in managing the data flow from online measurements. It enables real-time decision-making and trend analysis – both vital for process optimization and compliance with regulatory standards.
5. Cost and Time Efficiency. By automating the sampling and analysis processes, the Online LC system minimizes the need for manual intervention, thereby reducing labor costs and the potential for human error. It also speeds up the process development and scale-up phases by providing immediate feedback on process parameters.
These capabilities make Agilent’s Online LC solutions a valuable asset for bioprocessing applications, particularly in the production of mAbs, where quality and efficiency are paramount.