Temperature-induced unfolding of three humanized IgG1 monoclonal antibodies and their Fab and Fc fragments was monitored by differential scanning calorimetry at neutral pH. With some exceptions, the thermogram of the intact antibody presents two peaks and the transition with the larger experimental enthalpy contains the contribution from the Fab fragments. Although the measured enthalpy was similar for all three Fab fragments studied, the apparent melting temperatures were found to vary significantly, even for Fab fragments originating from the same human germline.
Choosing the best antibody to progress in your biologic pipeline
Introduction
The rapid growth of the therapeutic antibody market has increased the interest in modeling of the antibody structure and in understanding the factors that affect the function and the stability of antibodies. As of 2006 there were 18 monoclonal antibodies approved for therapeutic use in US, and 14 of them were molecules of the IgG isotype.1 Among these, 50% are humanized IgG1 anti-bodies. The antibody molecule (Figure 1) is formed by two identical heavy chains (~450 residues each) and two identical light chains (~220 residues each).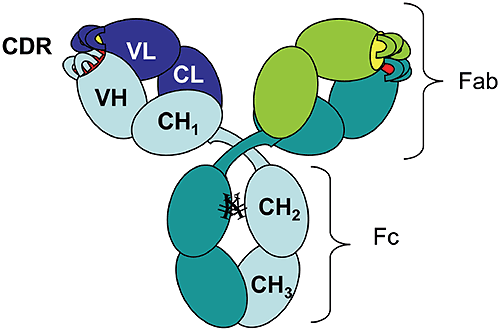
Figure 1. Schematic diagram of an IgG1 molecular structure: VH, variable heavy chain domain; CH, constant heavy chain domain; VL, variable light chain domain; CL, constant light chain domain; CDR, complementarity determining region. ¥ represents carbohydrates covalently linked to Asparagine residues. The chains fold into domains, of about 110 residues, that have a characteristic beta-sheet architecture known as the "immunoglobulin fold." There are two immunoglobulin domains in the light chain, VL and CL, and four immunoglobulin domains in the heavy chain, VH, CH1, CH2, and CH3 ("V" and "C" stand for "variable" and "constant," respectively). Digestion of human IgG1 antibody by papain results in two types of fragments of similar size: the Fc fragment (composed of two CH2 and two CH3 domains) and the Fab fragment (composed of CH1, CL, VH, and VL). Mouse-derived antibodies are often subject to immune recognition when administered to humans and their effector functions governed by species-specific Fc fragments are diminished. This has been overcome by "humanization" of murine antibodies, which involves the transfer of complementarity determining regions (CDRs) from the murine antibody along with some adjacent framework regions into the variable domains of the heavy and light chains of a human antibody.2 To identify the key residues to be transferred with CDRs, computer models are used to generate the 3-D structure of the variable domains that can incorporate the CDRs, while preserving the binding specificity and affinity for the desired target.3 There is a limited number of existing human variable domain frameworks (coded in the germline),4 and the selection of the framework is done based on the sequence homology with the murine antibody.2 Temperature-induced unfolding of monoclonal antibodies measured by differential scanning calorimetry (DSC) has become an indispensable tool used for monitoring production consistency, clone selection, protein structure characterization, and may potentially have a value for formulation development. Consequently, correct interpretation of the observed transitions is essential. Temperature or pH-induced unfolding of intact antibodies has been studied, however, mostly with antibodies of non-human origin, such as murine5-8 or rabbit9,10 immunoglobulins. Temperature-induced unfolding of human IgG1 and its Fab and Fc fragments was studied with the myeloma IgG1 Van by DSC in the pH range 3.5-5.5 and compared to the thermograms obtained from other human isotypes (IgG2, IgG3, and IgG4).11 The unfolding of the human myeloma IgG1 at pH 5.5 presents two transitions with the melting temperatures (Tm) around 70°C and 82°C, and with the amplitude of the first peak much larger than that of the second peak. Studies on the Fab and Fc fragments of the same antibody reported a melting temperature of 70°C for the Fab fragment11 and melting temperatures of 66°C and 82°C for the Fc fragment.12 Based on these results one may infer that the first unfolding event in the intact human IgG1 antibody is associated with the melting of the CH2 domain in the Fc fragment and the melting of the Fab fragment, while the second transition represents mainly the unfolding of the CH3 domain. Analogous studies using murine antibodies and their corresponding Fc and Fab fragments7,8 presented a similar trend in the unfolding events, with the unfolding of the Fab fragment occurring at lower temperatures than that of the Fc fragment. Using three different humanized IgG1 antibodies we show here that different melting profiles can be observed depending on the stability of the Fab fragment. We also demonstrate that the stability of the Fab fragment is significantly affected by the sequence of the variable domains. Therefore, one cannot assign a priori the first transition observed by DSC of the intact antibody to the unfolding of the Fab fragment; rather it is the experimental enthalpy of unfolding, as determined by the peak area in the DSC thermogram, which may be used as the indication for which transition represents the Fab fragment unfolding. This approach relies on the assumption that the Fab fragment unfolds in a cooperative manner, that is, only one transition is observed in the thermogram of the Fab fragment. If the coupling among the domains in the Fab is disrupted by CDR grafting and humanization process, the thermogram of the Fab fragment may present multiple transitions, and the interpretation of the DSC profile for an intact antibody will become more complex. As will be shown below, multiple transitions observed in Fab unfolding should be carefully examined because may represent artifacts of papain digestion. One has to emphasize that the amplitude criterion can be used for DSC measurements only, and cannot be extended to other methods which track the temperature-induced unfolding by a spectroscopic signal (like circular dichroism or fluorescence).
Experimental section
Protein preparation One humanized antibody of type IgG1k was produced and purified in Merck Research Laboratories, and will be further referred as monoclonal "Mrk." The other two humanized antibodies, trastuzumab (Herceptin®) and bevacizumab (Avastin®), are produced by Genentech and are commercially available. The latter monoclonals will be further referred to as "Her" and "Ava," respectively. Unless otherwise stated, the proteins were dialyzed prior to DSC measurements against the same buffer containing 10 mM sodium phosphate, 150 mM sodium chloride at pH 6.5. The protein concentration of intact antibodies or Fab and Fc fragments was measured in 6 M Guanidine Hydrochloride (GuHCl) spectrophotometrically. The extinction coefficient at 280 nm was calculated for each protein based on the content of tryptophans, tyrosines, and cystines.13 Papain digestion and fragment purification Monoclonal antibodies were digested with immobilized papain (Pierce kit cat #44885, Rockford, IL) following manufacturer’s instructions, with the exception that the incubation at 37°C was carried overnight. For Mrk and Ava antibodies, the Fab and Fc fragments were separated using Protein A column as described in the kit. Her antibody Fab fragment was found to bind to Protein A (it eluted from protein A column in the Fc fraction) and, therefore size-exclusion chromatography (SEC) was used for fragment separation. SEC was carried out on a ProStar HPLC system (Varian, Walnut Creek, CA) using two G3000SWXL columns (Tosoh Bioscience, Tokyo, Japan) connected together. The mobile phase used was 25 mM sodium phosphate, 150 mM sodium chloride, pH 6.8 and the flow rate was 0.5 mL/ min. With papain digestion carried overnight the digestion of Mrk antibody was not complete. Therefore, the Fc fragment of Mrk antibody was further purified using SEC (the same as above) to eliminate the undigested and partially digested (Fc-Fab) antibody. Mass spectrometry together with SEC and SDS-PAGE revealed that approximately 75% of the Ava Fab fragment sample contained one or two clips in the VH domain and, importantly, that these clipped molecules are in a dimeric form (data not shown). Ava antibody Fab fragments, intact or clipped, were separated using the SEC system described above. The intact fraction of Ava Fab sample, purified by SEC, had 95% purity. Mass spectrometry confirmed that the intact fraction is formed by an intact light chain and an N-terminal heavy chain fragment that resulted from the expected papain digestion at H/T position in the hinge region, without additional cleavage sites in the VH domain. The Fab fragment from the Ava antibody, containing intact and clipped molecules, was found to have a broad, two-peak transition by DSC, suggesting that there are at least two distinct species with different melting temperatures. Based on the DSC profiles of the purified clipped and intact Fab fragments (data not shown), we propose that the transition with the lower melting point in the dimer(clipped)/ monomer (intact) mixture represents the dissociation/unfolding of clipped monomers. It is possible that the dimers, held together by non-covalent interactions, dissociate at higher temperatures and the clipped monomers are destabilized compared to the intact monomer due to the disruption of the polypeptide chain. Protein deglycosylation Two milligrams of Mrk antibody was mixed with 2.2 ml of peptide N-glycosidase F (PNGase F, New England Biolabs, Ipswich, MA) and 20 ml of G7 buffer provided with the enzyme. The digestion was carried out for 24 h at 37°C. PNGase F converts the asparagine residue that contains the glycan chain into aspartic acid, and the completeness of the deglycosylation can be monitored by cation-exchange chromatography (CEX). CEX was performed with ProPac WCX-10 4 250 mm column (Dionex Corporation, Sunnyvale, CA). Ten millimolars sodium phosphate pH 6.5 was used as mobile phase A. Mobile phase B had the components of mobile phase A plus 0.5 M NaCl. The antibody was separated with 10-45% gradient B over 25 min at 0.5 mL/min flow rate. Size-Exclusion Chromatography (SEC) with on-line refractive index and light scattering detection The Fab fragment from Ava antibody sample was separated by SEC on Waters 2690 Separations Module (Waters Corporation, Milford, MA) using a G3000SWXL column (Tosoh Bioscience) with online refractive index (RI) (Waters 2410 RI Detector) and light scattering (LS) detection (PD2000, Precision Detectors, Franklin, MA). The mobile phase was 150 mM sodium chloride at a flow rate of 0.5 mL/min. The ratio of the refractive index and light scattering signal was used to calculate the ratio of molecular weights of the two Ava antibody Fab fragment fractions as described previously.14 Shortly, RI and LS signals were normalized by the height of the putative dimer peak. The LS/RI for the maximum of the monomeric peak and the monomeric peak area were calculated to be 0.53 and 0.49, respectively. Because the LS/RI ratio is proportional to the molecular weight of the species, the putative dimer was found to have twice the molecular weight of the monomer. SDS-PAGE SDS-PAGE was performed using Tris-Glycine 4-20% gel (Invitrogen, Carlsbad, CA). Samples were prepared according to manufacturer’s instructions and 15 mg of the Ava antibody Fab fragment were loaded per lane. Mass spectrometry Mass spectrometry analysis was performed using a capillary HP1100 HPLC system coupled to an Agilent MSD-ESI-TOF VL mass spectrometer. In order to examine the masses of the light and heavy chains, the Fab samples were incubated with 50 mM DTT for 15 min at 75°C, followed by dilution into formic acid. The samples were injected onto a 0.5 mm50 mm Monolithic PSDVB column (Dionex), equilibrated at 70°C and eluted with a gradient of 0.1% formic acid in water and acetonitrile at a flow rate of 0.02 mL/min. Mass reconstruction from the raw data was accomplished with the Protein Confirmation software provided with the system. DSC measurements The DSC measurements were performed, unless otherwise mentioned, at 1 mg/mL protein and 1°C/min scan rate on a MicroCal VP-Capillary DSC Platform (MicroCal, LLC, Northampton, MA). The DSC profiles were calculated using the Origin 7.0 software: the buffer background was subtracted and the thermogram was normalized to the molar concentration of the protein. The final excess heat capacity thermogram was obtained by interpolating a cubic baseline in the transition region. The melting temperatures reported represent peaks in the experimental thermograms and the enthalpy of unfolding was obtained using the Origin 7.0 software by integration of the area under the melting curves in the temperature range 55–90°C. Because the transitions reported in this study are irreversible, all the experimental values reported for melting temperatures and enthalpies should be regarded as "apparent" values.Results
Temperature-induced unfolding of intact antibodies The profiles of temperature-induced unfolding of three humanized IgG1 monoclonal antibodies (Mrk, Her, and Ava), under the same solvent conditions, are shown in Figure 2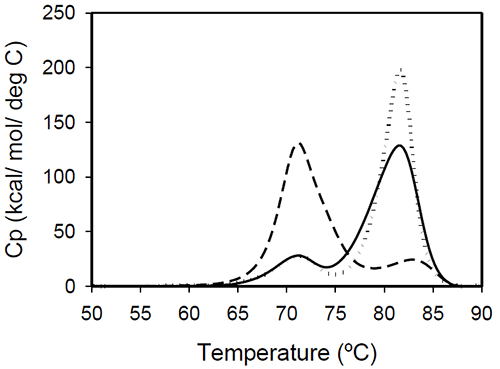
Figure 2: Temperature-induced unfolding of full length IgG1 monoclonal antibodies: Mrk (solid line), Her (dotted line), Ava (dashed line). All proteins were in 10 mM sodium phosphate, 150 mM NaCl, pH 6.5. The thermogram of each protein consists of two transitions, centered around 70 and 80°C, but with different corresponding apparent enthalpies. For the Ava antibody, the first transition has larger amplitude than the second transition, while for Mrk and Her antibodies, the second transition has the larger amplitude. To evaluate the errors in determining melting temperatures and enthalpies, duplicate measurements for each antibody were performed several weeks apart. The results indicate that the variation of the melting temperature is within 0.25°C and that the variation in the measured enthalpy is less than 3.5%. The DSC profile observed for the Ava antibody is similar to the temperature induced unfolding of another human IgG111 and a murine IgG2a antibody.7 Two transitions were observed for the murine antibody, with the first peak area about twice the area of the second peak. Further studies with fragments of the murine IgG2a antibody clarified that the first transition corresponds to the Fab fragment unfolding, while the second transition corresponds to the melting of the Fc fragment. Similar trend was reported for a different murine IgG2 antibody and its Fab and Fc fragments:8,15 between pH 6 and 8 the intact antibody presents two transitions, with Fab unfolding corresponding to the first transition and Fc unfolding representing the second transition. In contrast, for a murine IgG1 antibody, only 1 peak was observed at pH 6.0,6 the difference from IgG2a being ascribed to differences in the flexibility in the hinge region between isotype 1 and 2 in mice. Among the three humanized IgG1s investigated in this study, the temperature-induced unfolding of the Ava antibody seems to follow the pattern observed for the two murine IgG2 antibodies. However, it is obvious that this trend is not followed by other humanized IgG1 monoclonal antibodies and, as it will be demonstrated later, the interpretation of the murine thermogram is not applicable to humanized antibodies. Detailed analysis of Fab and Fc fragments of humanized IgG1 antibodies described below shows a clear distinction between the thermograms of the Ava and murine IgG2 antibodies: the first transition in the Ava antibody represents the unfolding of the Fab fragment and the unfolding of one domain in the Fc fragment, while only the unfolding of the Fab fragment contributes to the first transition of the murine IgG2 antibodies reported so far. Temperature-induced unfolding of fab fragments Fab fragments from Mrk, Her, and Ava antibodies were obtained by papain digestion followed by chromatographic separation using protein A and/or size-exclusion columns. The purity of the Fab fragments, assessed by SEC and non-reduced SDS-PAGE was ~90% for the Her antibody, and larger than 95% for Mrk and Ava antibodies (data not shown). Temperature-induced unfolding profiles of Fab fragments from Mrk, Her, and Ava antibodies are shown in Figure 3.
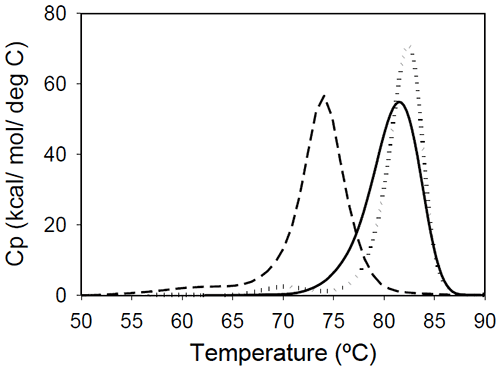
Figure 3. Temperature-induced unfolding of Fab fragments of IgG1 monoclonal antibodies: Mrk (solid line), Her (dotted line), Ava (dashed line). All proteins were in 10 mM sodium phosphate, 150 mM NaCl, pH 6.5. The thermograms of Mrk and Her Fab fragments present only one peak around 80°C, the temperature where the large-amplitude transition was observed for the corresponding intact antibodies. For both Mrk and Her Fab fragments the experimental enthalpies of unfolding are in the range 320–340 kcal/ mol. For each fragment, the variation in the measured enthalpy was found less than 3% and the variation in measured Tm<0.2°C. The Fab fragment from Ava antibody was found to show structural heterogeneity after papain treatment (see Experimental section for more details). Additional papain cleavage sites in IgG1 variable domains were reported and it has been proposed16 that CDR conformation could be the determining factor for the increased susceptibility for enzymatic digestion. The DSC profile of the intact Ava Fab fragment (purified from the heterogenous sample by SEC) is shown in Figure 3 and it has a melting temperature of ~74°C, close to that observed for the first transition in the full-length Ava antibody (Figure 2). The experimental enthalpy of unfolding of the Ava antibody Fab fragment is 316 kcal/mol, within 1% of the experimental enthalpy of the Her antibody Fab fragment. It is worth noting that the clipped Fab fragment of Ava antibody is significantly destabilized compared to the intact fragment and has a strong tendency to dimerize. Based on the concentration dependence (data not shown), the dimers formed upon papain cleavage are distinct from dimers previously reported for the intact Ava antibody.17 >> Download the full Application Note as PDF
Malvern provides the materials and biophysical characterization technology and expertise that enables scientists and engineers to investigate, understand and control the properties of dispersed systems. These systems range from proteins and polymers in solution, particle and nanoparticle suspensions and emulsions, through to sprays and aerosols, industrial bulk powders and high concentration slurries. Used at all stages of research, development and manufacturing, Malvern’s instruments provide critical information that helps accelerate research and product development, enhance and maintain product quality and optimize process efficiency. Our products reflect Malvern’s drive to exploit the latest technological innovations. They are used by both industry and academia, in sectors ranging from pharmaceuticals and biopharmaceuticals to bulk chemicals, cement, plastics and polymers, energy and the environment. Malvern systems are used to measure particle size, particle shape, zeta potential, protein charge, molecular weight, mass, size and conformation, rheological properties and for chemical identification, advancing the understanding of dispersed systems across many different industries and applications. www.malvern.com Material relationships http://www.malvern.com/en/ [email protected]

