Analytical results are the basis on which we make calculations and decisions, perform risk assessments, give recommendations, and identify problems. But what if our analytical tools aren’t efficient enough to protect us? Food, cosmetics, and consumer goods are all potential sources of harmful compounds. For example, they contain oils with unsaturated fatty acids, which readily oxidize to hydroperoxides and epoxides with genotoxic effects. A powerful analytical tool that could detect these genotoxins would help us determine how best to process and store oils while keeping them stable in products.
There is a gap in our knowledge of the foods we eat and the cosmetics we use because of the difficulty in comprehensively analyzing the thousands of different compounds they contain. Researchers often discuss which analytes to focus on or which structures to elucidate – so, even for known target substances, not all degradation products and metabolites are always known depending on the surrounding matrix and processing, and so on. In particular, powdered foodstuffs (such as spices, dietary supplements, botanicals and colored food extracts) are vulnerable to adulteration and falsification due to the global production chain. Especially sustainable food systems are suspected of accumulating harmful compounds. In the past, falsification counterfeiting and adulteration was mostly uncovered by whistleblowing, which calls into question the efficiency of our current analytical tools.
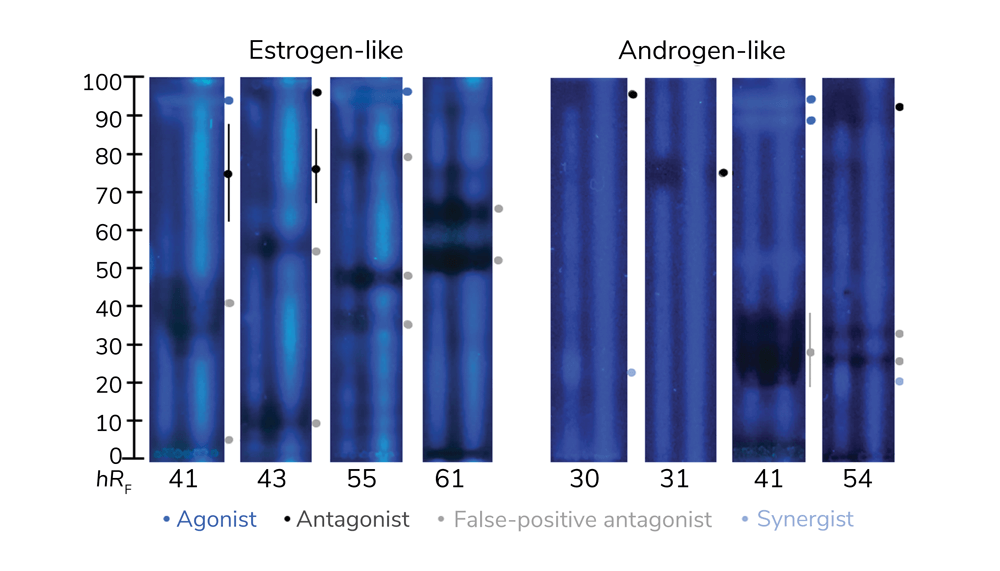
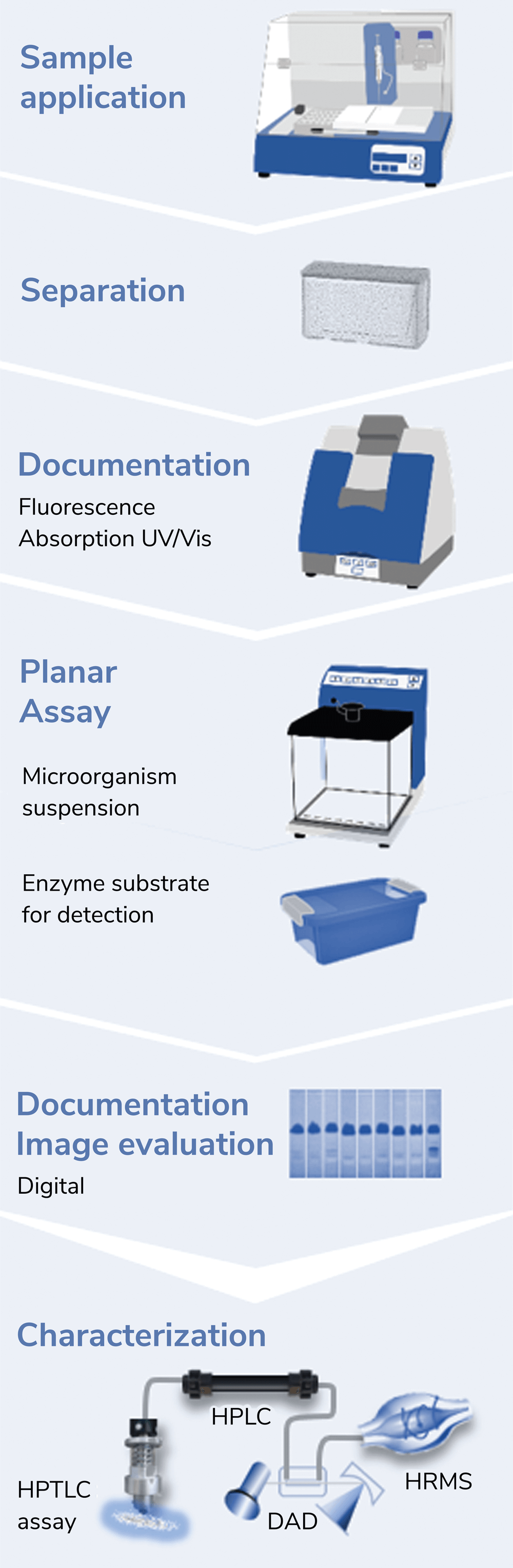
Recently, research has indicated that planar effect-directed on-surface assays are superior to microtiter plate assays (1). This is largely because these assays combine the power of two disciplines (chemistry and biology/toxicology). To perform the technique, many samples in parallel are separated into zones, then assayed on the same surface for harmful biological effects (of course, beneficial effects can also be detected depending on the assay). This prioritizes the most important active compounds and avoids intensive sample preparation, which often alters the sample composition. Sample integrity is important because, to ensure comprehensive analysis, no part of the sample should be lost. Applying planar assays to food and cosmetics screening increases the likelihood of finding genotoxic (Figure 1) or endocrine active substances (Figure 2) present in the samples (2-4).
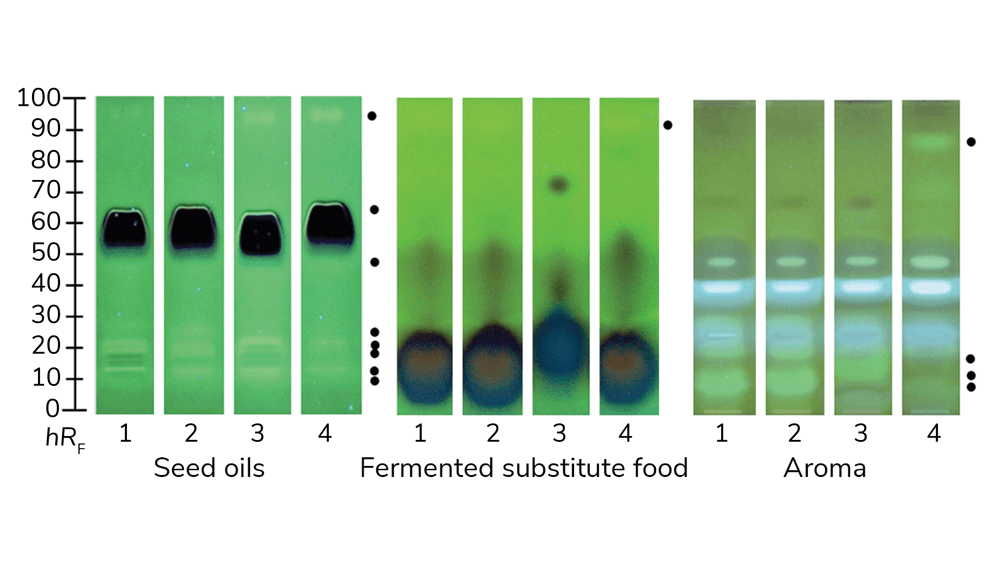
Current analytical techniques fall short because researchers only look for particular target compounds, or because potentially important signals get lost in the thousands of unknown compounds. If one views all the positive and negative signals of a sample track in an in vitro microtiter plate assay as sum value, the issue becomes obvious: weaker genotoxic signals are easily overlooked in complex mixtures or disappear in the quenching effect of strongly absorbing substances or in the antagonistic effect. This is because the crucial chromatographic separation is missing. Only in previously separated samples are signals differentiated and clearly visible.
Incorrect analytical results lead to incorrect conclusions, and inadequate tools hinder our understanding. Differentiating between effects (Figures 1 and 2) is crucial for reaching airtight conclusions. The open format of planar separation enables the application of two stripes to each separated sample track, which, after the bioassay, allow the simultaneous detection of multiple effects (Figure 2). The first (agonist) stripe detects antagonistic substances by fluorescence signal reduction; the second (end product) stripe detects false-positive antagonists by physicochemical fluorescence quenching. Synergists enhance the blue fluorescence on the first agonist stripe. We can therefore differentiate between agonistic, antagonistic, false-positive antagonistic, and synergistic effects in a single assay. Dose-response curves can be calculated through digital image analysis. To approximate one multiplex bioautogram, four status quo in vitro assays would be needed per sample. Therefore, multiplex on-surface assays (3, 4) offer a great advantage in analyzing complex samples.
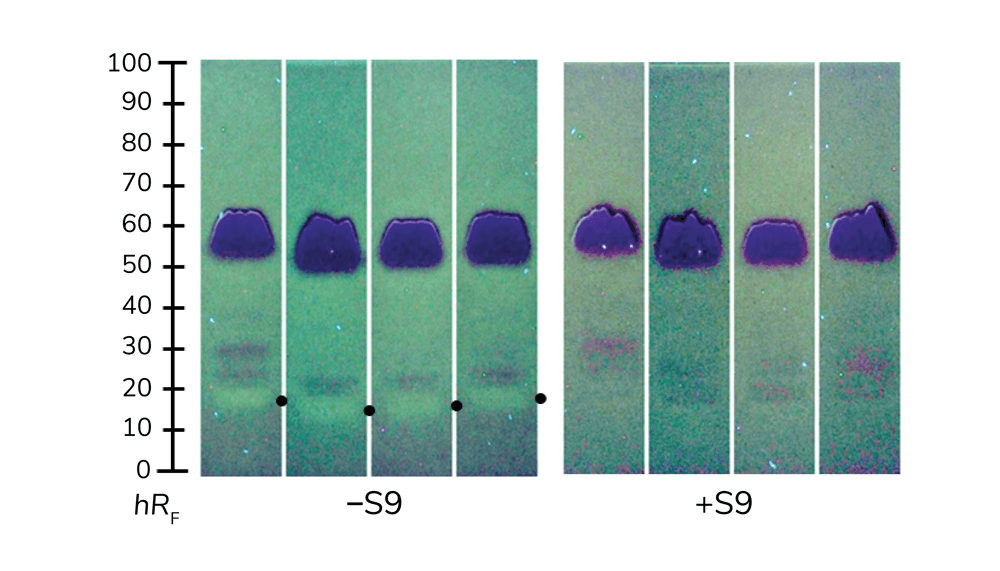
Our bodies can metabolize and, in the best case, detoxify harmful substances in the liver (if it is in good working order and no damage occurs before the toxins reach the liver). Conversely, liver metabolism can also make substances more toxic by converting precursor molecules into their active forms (2). On-surface metabolization using planar assays can help identify which substances may have which effects (Figure 3).
In vitro microtiter plate assays are blind to opposing effects or opposing signal responses in complex samples and often give false values. Although non-target approaches such as spectral fingerprinting and chromatography-mass spectrometry coupling techniques open new vistas, they do not prioritize the important active compounds. Multitarget approaches cover several hundred analytes, but can never be comprehensive. So how can we quickly find important (hazardous) compounds – especially ones that have not previously been a focus?
A shift toward planar assays would detect harmful compounds and provide better overall safety. The planar screening technique’s validity has been demonstrated and it is ready for use (Figure 4). Planar effect-directed screenings require only five to 20 minutes per sample analysis and have consumption costs of €0.50−1.00 per sample. Investment costs for commercial systems are approximately €70,000, whereas an open-source, do-it-yourself system (5) requires only about €1,700 for material costs. Self-assembly allows users to become familiar with portable, small-scale systems and facilitates self-repair. If harmful zones are detected in a complex sample and cannot be assigned to known compounds, high-resolution mass spectra can be recorded directly from the bioautogram (3, 4, 6). The optional superhyphenation makes the on-surface assay strategy highly efficient. A next training course on planar assays will take place digitally in March 2023 (www.uni-giessen.de/food).
References
- G Morlock, Analytica Chimica Acta, 1180, 338644 (2021) DOI: 10.1016/j.aca.2021.338644.
- G Morlock, D Meyer, Food Chem, 408, 135253 (2022). DOI: 10.1016/j.foodchem.2022.135253.
- A Ronzheimer et al., Phytomedicine, 250, 154230 (2022) DOI: 10.1016/j.phymed.2022.154230.
- T Schreiner et al., Food Chem, 395, 133610 (2022) DOI: 10.1016/j.foodchem.2022.133610.
- L Sing et al., Anal Chem, 94, 14554–14564 (2022) DOI: 10.1021/acs.analchem.2c02339.
- A Mehl et al., J Chromatogr A, 1651, 462334 (2021) DOI: 10.1016/j.chroma.2021.462334.