It is well documented that miniaturization of the separation medium offers several advantages for high-resolution LC-MS analysis of complex samples, such as those encountered in proteomics, metabolomics, and biopharmaceutical characterization. The inherent high permeability and low on-column dispersion obtained by the perfect order of the separation bed in micro-pillar array columns (µPAC) is superior to packed beds and monoliths for high-resolution separations. We have recently illustrated this in a number of applications using the first generation of commercially available µPAC to analyze biopharmaceuticals using µPAC combined with mass spectrometry (µPAC-MS), described below. Moreover, the µPAC concept can in the future be tuned and optimized for production of separation media with unique features for high throughput and high productivity applications as well … to follow!
Characterizing antibody-drug conjugates
Kadcyla (ado-trastuzumab emtansine) and, for comparison, Herceptin (trastuzumab) tryptic digests were analyzed on a 200 cm µPAC C18 column and peaks eluting were detected by UV spectroscopy and high-resolution MS. The resolving power of the µPAC column allows an in-depth study of conjugation sites and the separation of isomeric conjugated peptides.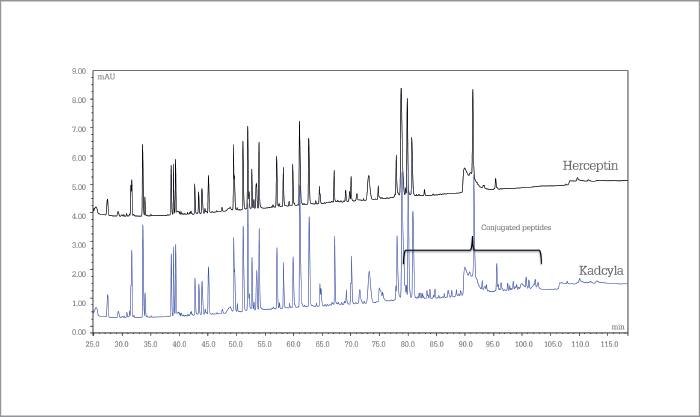
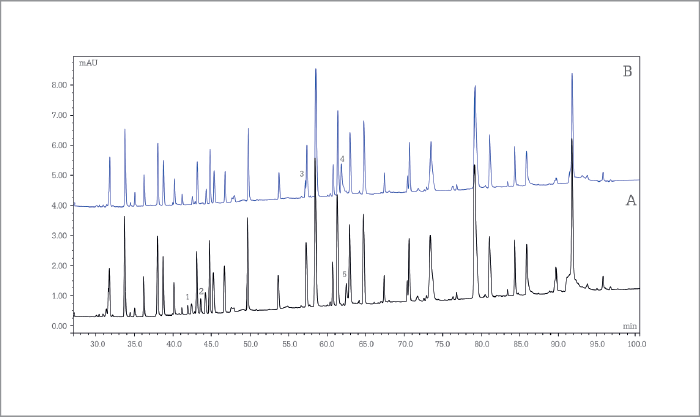
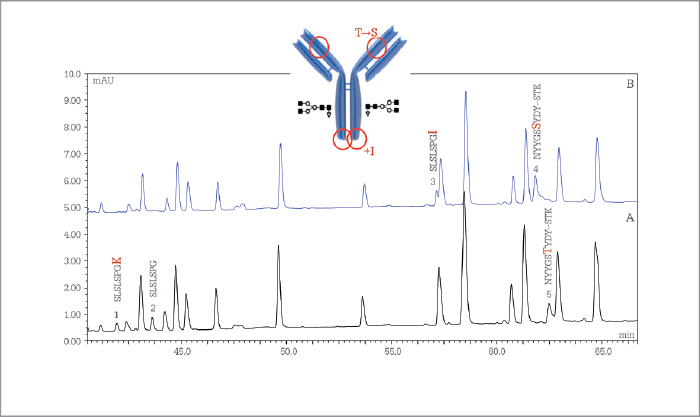
Monoclonal antibody peptide mapping
Remicade (infliximab) originator and candidate biosimilar tryptic digests were analyzed on a 200 cm µPAC C18 column and peaks eluting were detected by UV spectroscopy and high-resolution MS. The simultaneously acquired MS and MS/MS data enabled us to identify differences between the originator and candidate that rule out the candidate for further development.