Give us a snapshot of mass spectrometry (MS) imaging Axel Walch: From a clinical perspective, MS imaging – unlike most imaging methods – removes the need for target-specific labeling whilst measuring the required broad spectrum of endogenous and exogenous analytes in tissues within the histological context. The highly multiplexed analyses offered by MS imaging enables not only diagnostic (targeted) assays but also the potential for biomedical discovery applications. Multi-class analysis capability (from proteins and peptides to drugs, lipids and beyond) is a further strength of MS imaging. The practical simplicity of MS imaging and its ability to gain reliable information, even from the smallest tissue samples, means that it has the potential to complement traditional histopathologic evaluation for assisting in diagnosis, risk assessment, or response prediction to therapy. Ron Heeren: Right now, there is a strong push for higher information content imaging to unravel the molecular complexity of biological surfaces. This includes a drive for higher resolution techniques – both higher spatial resolution and higher molecular resolution. The latter is being pushed by high-resolution mass spectrometry (MS) techniques (Fourier transform (FT)-MS, TOF systems and so on), as well as the incorporation of ion mobility separation to enrich the wealth of molecular information obtained from complex organic surfaces. Another important development is the move towards rapid quantitative MS imaging strategies that use selective reaction monitoring (SRM) approaches and dedicated internal standards. In particular, this is a requirement for pharmaceutical applications, such as pharmacokinetic and pharmacodynamic (PK/PD) studies. Ambient imaging technologies are opening up a whole new area of MS imaging. Essentially, samples that are not vacuum-compatible can now be imaged at the molecular level. In some cases, such as with laser ablation electrospray ionization (LAESI), multiply-charged ions can be produced, and that offers various analytical advantages. This step change now allows true top-down imaging to be developed.
What trends do you see in the application of MS imaging?
RH: MS imaging is developing into an analytical tool for the fundamental discovery of signaling pathways of disease, leading to validated molecular disease profiles in the area of clinical molecular imaging. These disease profiles can be used for staging a disease, that is, for diagnostic and prognostic purposes. Even more importantly, the breadth of molecular information that MS imaging offers is a key input parameter for personalized medicine. In reality, it is rare to find a single molecule or biomarker that can be used to determine the course of a disease. Rather, it is an understanding of the interplay between proteins, lipids and other small molecules that offers the true solution, and that is exactly what MS imaging offers. It is this that will define its role in the future.
The heterogeneity of tumors can be fully disclosed using MS imaging. Lipids, primary metabolites and peptide/protein images reveal the tumor margins and provide insights into molecular signaling pathways. This makes MS a key tool for various aspects of oncology, such as identfying surgical margins, developing personalized therapies and tumor staging.
Other areas of medicine will also benefit from the fundamental understanding of the spatial molecular composition. One example is cardiovascular research: atherosclerotic plaques and the interaction of stents with arterial walls can be much more fully explored. Another is neurodegenerative diseases: altered molecular signals that lead to tissue loss or the formation of protein plaques in the brain can be dissected. The power to examine all molecules simultaneously will provide true mechanistic understanding of disease.
In the more distant future, I predict that there will be discovery-based approaches, drug distribution and effect monitoring… possibly even MS imaging within the
GP’s office.
AW: To answer that, let me offer a brief description of the current status of pathology in personalized medicine. The task of the pathologist is to assist physicians in the correct diagnosis of diseases at the earliest possible stage; this allows the optimal treatment strategy to be developed for each patient. Surgical pathology (the traditional tissue diagnosis of biopsies or surgical resection specimens) is simply a tool based on histology. “Molecular morphology” aims to combine novel molecular and genetic information with the well-established histomorphologic features that continue to define cancer and many other types of diseases mentioned by Ron. Certain molecular and genomic technologies for tissue analysis are already useful in diagnosis of disease, taking us towards treatment tailored to individual patients. Personalized medicine – the future of patient health care – demands personalized pathology. This would integrate the flood of new molecular data with traditional surgical pathology, digital histopathology, and clinical data in electronic medical records. MS imaging of tissues has a central role in combining traditional tissue diagnosis by histopathology with highly multiplex molecular analysis. MS imaging data sets are enormously complex, comprising thousands of unique ion images. These images can be registered to high-resolution digitized histology images, correlating molecular information and adding a new dimension of understanding to the histopathology and thus disease.
The future for personalized pathology is that molecular signatures of proteins, peptides, lipids, and molecules of cell metabolism obtained by MS imaging directly from patient tissues, will improve diagnosis and therapy response prediction for improved patient stratification. In PK/PD, the application of MS imaging to determine the tissue distribution of drugs and their metabolites will have a dramatic impact on both drug discovery and development.
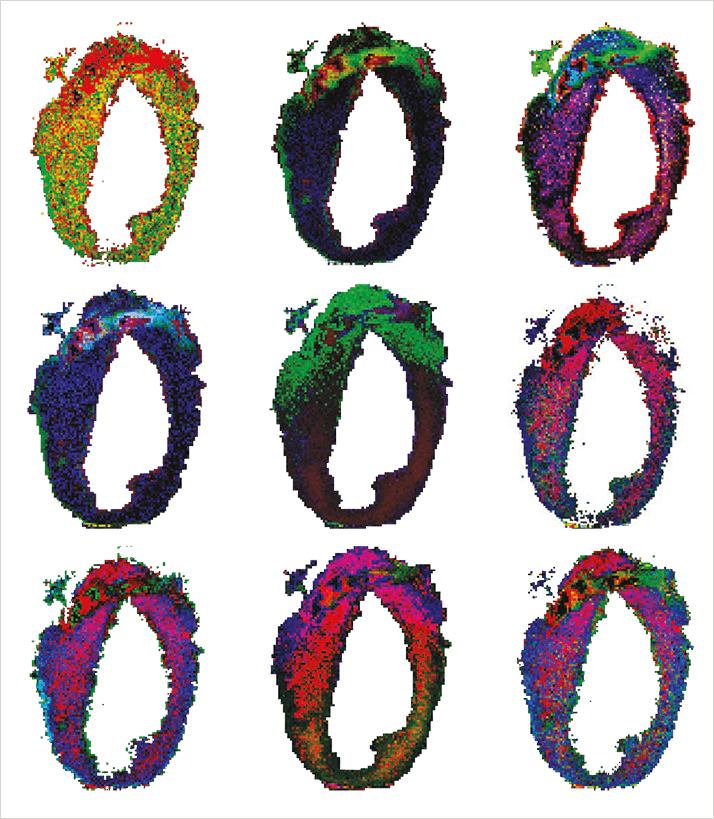
What have been the biggest MS imaging breakthroughs of the last decade? RH: The birth of atmospheric desorption and ionization techniques and their combination with mass spectrometry have opened up a new field of imaging and analysis. This has allowed analysis to be performed in situ, for instance during surgery and has also enabled researchers to study non-vacuum-compatible samples. Another huge advance was the recent development of top-down imaging MS in which large proteins are localized and, importantly, identified in the same high resolution MS experiment. The recent application of active pixel detectors coming from high-energy physics has increase throughput and sensitivity. In the secondary ion mass spectrometry (SIMS) community, new particle beams (C60, Argon and H2O clusters) have enabled detailed depth profiling and 3D imaging studies with larger intact molecular species. All very exciting breakthroughs. AW: From a preclinical and clinical research point of view, there has been a plethora of methodological publications about MS imaging, but only a limited number of studies clearly demonstrated an impact on MS imaging in biomedical and clinical research applications – my main area of interest. The following impressive highlights show the potential for biomedical discovery:
- Imaging mass spectrometry: a new technology for the analysis of protein expression in mammalian tissues (1)
- Mammalian heart renewal by pre-existing cardiomyocytes (2)
- Ambient mass spectrometry for intraoperative molecular diagnosis of brain tumors (3)
- Imaging mass spectrometry reveals modified forms of histone H4 as new biomarkers of microvascular invasion in heptocellular carcinomas (4)
- Chemo-informatic strategy for imaging mass spectrometry-based hyperspectral profiling of lipid signatures in colorectal cancer (5).
What are the greatest challenges facing MS imaging? AW: MS imaging technology has huge potential, but do we want to see improved spatial resolution, the detection of higher molecular weight compounds, greater sensitivity, better quantitation, and increased molecular coverage? Of course we do! And, as with other analytical techniques that have made the leap, increased system stability, reproducibility, and user-friendliness will be essential, if we are considering MS imaging for routine use in a clinical setting. This is where vendors can help; to ensure a successful transition, fully integrated systems are an absolute must. Some are moving in the right direction, but a complete solutions platform – from sample prep to MS analysis to bioinformatics – is not yet available. I would love to know what’s in the pipeline… RH: While the technology is evolving rapidly and resolution and speed are improving annually, challenges still exist. Research is striving for ever more detailed insights but we are falling orders of magnitude shy of the challenges in bioinformatics and clinical validation of results. Standardization and validation are two issues that must be addressed for clinical maturation. More and more data is being generated using protocols that are fit-for-purpose rather than being standardized, making it difficult to generate large-scale, validated e-biobanks with assured quality. There is a clear need for standardization of protocols and tissue standards to evaluate the quality of the data being entered into the databases. The Human Tissue Atlas is a perfect example of how immunohistochemistry (IHC) images are consolidated around different tissues and diseases. Something similar for MS tissue imaging would be an incredible clinical resource. The projection – or fusion – of MS images onto existing tissue atlases is another challenge that needs to be (and is being!) tackled by the MS imaging community. One example is the Allen Brain Atlas (www.brain-map.org), currently used by researchers involved in brain tissue imaging with MS. European network initiatives, such as the Cooperation in Science and Technology (COST) action on MS imaging, have the potential to address these challenges.
Clearly, you both envisage a strong role for MS imaging in a clinical setting… RH: The vast amount of molecular information generated by multimodal tissue imaging experiments is ideal for generating biobanks for different diseases. MS delivers a lot of immediate information on many different levels which can be used to make, for instance, well-informed clinical decisions during surgery or selecting personalized therapies. However, as stated earlier, this is only possible if the clinician can assess the information in context or immediately compare it to molecular disease profiles obtained from existing knowledge stored in an appropriate biobank or database. Once this infrastructure is established, the clinical diagnostic and prognostic applications of MS imaging will bloom. Biobanks will quickly be generated by analyzing large amounts of tissues and profiling approach (a concise set of quick local analyses) will be used to personalize therapy or adapt surgical procedures. AW: MS imaging does have huge clinical potential, especially in surgical pathology but also in other areas. I agree with Ron’s remarks on the need for development, standardization, and validation. Take the example of sample preparation, something we all hope to spend less time and effort on. While certain solutions have been developed, for example, pre-coated slides, spotting robots and spray coaters, much less has been done to standardize usage across laboratories or platforms. In order for diagnostic assays to become routine:
- Validated methodologies (for a statistically significant sample set) must be developed
- Methods for analyte identification must be improved
- Assay specificity must be confirmed (identification of the analyte in situ provides final confirmation that the assay sufficiently specific).
Can you comment on quantitative aspects of MS?
AW: Improvements are essential. While qualitative assessment can distinguish disease states based on the presence or absence of certain molecules, increasing numbers of applications require the ability to discriminate between subtle changes in analyte abundance. In these cases, we need absolute quantitation to make a valid clinical decision. This will require improvements to sample preparation, such as the addition of standards to the tissue, alongside increased instrumental range and more robust data analysis to advance MALDI imaging quantitation.
For early drug discovery, quantitative MS imaging could be deemed to be adequate. However, making accurate quantitative judgments (on a pixel-by-pixel basis) is some way off and must be addressed.
From a data analysis point of view, a number of software packages are attempting to address the time-consuming nature of both analysis and calibration. This will have a positive impact on the potential of quantitative analysis. Validation will be key to unlocking the true applicability of quantitative MS imaging – robustness is of
paramount importance.
RH: The quantitative aspects of many MS-based surface analytical techniques are understudied. We still do not have sufficient insight into why certain molecules ionize well in one tissue environment but not at all in another. This mechanism is referred to as tissue suppression or, more colloquially, “the matrix effect.” That’s a term well known by those using other MS analyses, employed here to indicate that we have few clues as to what determines which molecules we can and cannot image with MS based microscopes. The difficulty can, in part, be ameliorated by the use of clever internal standards or multiple reaction monitoring (MRM)-based approaches. Forensic applications are beginning to benefit from this.
However, there are still simply too many unknowns to make MS imaging quantitative and other targeted approaches currently offer a better solution when the compound of interest is known. In this light, MS imaging is a discovery tool for looking for the “unknown players” or the specific combination of molecules that make something happen in a living system.
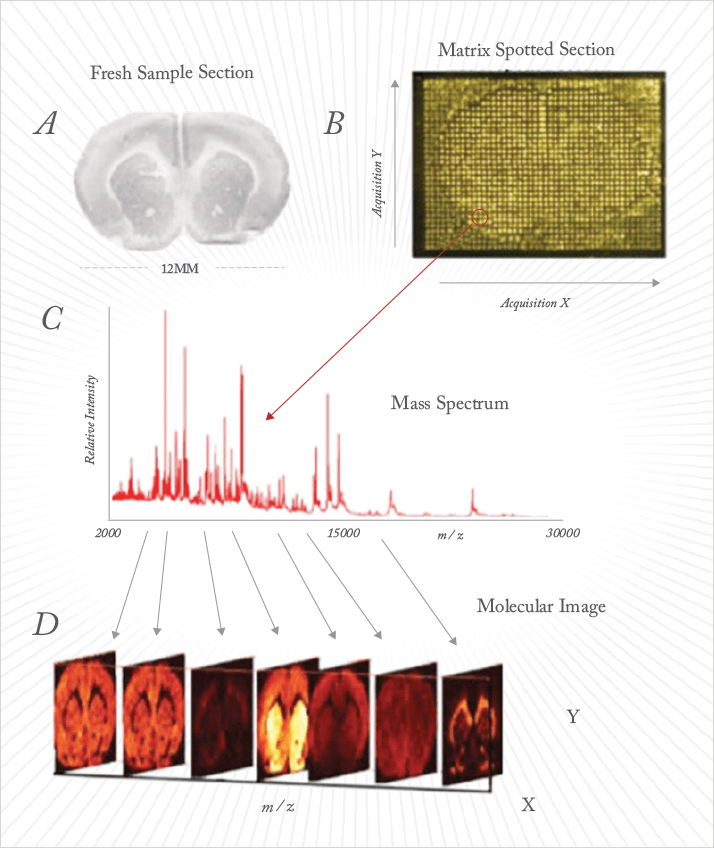
What is the most tantalizing analytical application? RH: From the many possible responses, I will offer three. One, in conservation science, MS imaging can be applied to the study of paint cross-sections. Two, investigations of bacterial colonies under environmentally-challenged conditions can be employed to investigate bioremediation of polluted environments. These studies can also offer insight into efficiently producing bio-fuels. And three, study of the interfaces between new biomaterials and living systems will enable better biocompatible materials and drug delivery systems to be designed. AW: The most tantalizing analytical applications of MS imaging are those that otherwise could not be performed directly with intact tissue in the natural histological context. Examples include the multiplex analysis of drugs, drug combinations and their associated metabolites; and imaging of cell metabolism molecules in situ, such as energy metabolism or the citric acid cycle. The simultaneous or sequential analysis of these different analytes on the same tissue section is possible, further increasing the uses of the technology. Such analyses at the level of individual cell populations within tissues could be not performed before the advent of MS imaging.
What specific choices need to be made to push progress? RH: We stand at a transition point. The technology has matured enough to be applied in the clinic, but small-scale efforts will lead to disappointments; a large-scale infrastructure is needed to take full advantage of the benefits. Investment is essential, as is the concerted effort of all involved. Large-scale molecular imaging centers, with participation of national science and national health councils will result in more efficient translational research. The imaging centers in Harvard and Vanderbilt universities are key examples that I hope Europe will follow, developing large-scale multimodal imaging approaches complemented by solid bioinformatics and e-biobanking infrastructure. All of this requires integration at the national and international level (EU/Horizon 2020 and WHO).
Lastly, talk us through the next decade AW: We’ve discussed challenges and limitations here. Despite these, MS imaging has seen exponential growth in both technological development and potential applications. I can only imagine it continuing to do so, joining a host of other established imaging techniques to form an ever more powerful toolbox combining broad coverage and exquisite specificity for the molecular analysis of tissues. MS imaging has already demonstrated its usefulness for biological discovery, and applications that display real clinical value are starting to surface more frequently. Given continued progress in the challenging areas mentioned above, MS imaging will increase its foothold in biomedical research and, as we move towards personalized medicine, begin to adopt its greatest role in personalized pathology. RH: New high throughput detectors, such as the IonPix detector from Omics2Image, and new high resolution imaging modes will be introduced. Parallel detection combined with imaging of increasingly large molecules or complexes will become possible and will take off with these new detectors, directly impacting the speed of analysis. In addition to new technologies, novel and efficient bioinformatics approaches are needed and, therefore, inevitable. All large-scale analytical data collected will be embedded in e-biobanks and made available to the larger medical community. This in turn will enable the introduction of small-scale MS-based analytical devices into health care systems. From here it is not hard to imagine a future where GPs have more informative tools at hand to quickly screen patients or determine drug levels and adjust therapy. Ultimately, this should reduce the cost of health care and improve quality of life for patients and their immediate environment.
Ron Heeren is group leader of Biomolecular Imaging Mass Spectrometry (BIMS) at Foundation for Fundamental Research on Matter (FOM) Institute AMOLF, Amsterdam, The Netherlands. Axel Walch is head of the Analytical Pathology research group, Helmholtz Zentrum München, Germany.
References
- Stoeckli et al., “Imaging mass spectrometry: a new technology for the analysis of protein expression in mammalian tissues”, Nat. Med. 7(4), 493-6 (2001). Senyo et al., “Mammalian heart renewal by pre-existing cardiomyocytes”, Nature, 493(7432) 433-6 (2013). DOI: 10.1038. Eberlin et al., Ambient mass spectrometry for the intraoperative molecular diagnosis of human brain tumors. Proc. Natl. Acad. Sci. USA. 110(5), 1611-6 (2013). Pote et al., “Imaging mass spectrometry reveals modified forms of histone H4 as new biomarkers of microvascular invasion in hepatocellular carcinomas”, Hepatology 58(3), 983-94 (2013). Veselkov et al., “Chemo-informatic strategy for imaging mass spectrometry-based hyperspectral profiling of lipid signatures in colorectal cancer”, Proc. Natl. Acad. Sci. USA (Jan 7, 2014: Epub ahead of print).




