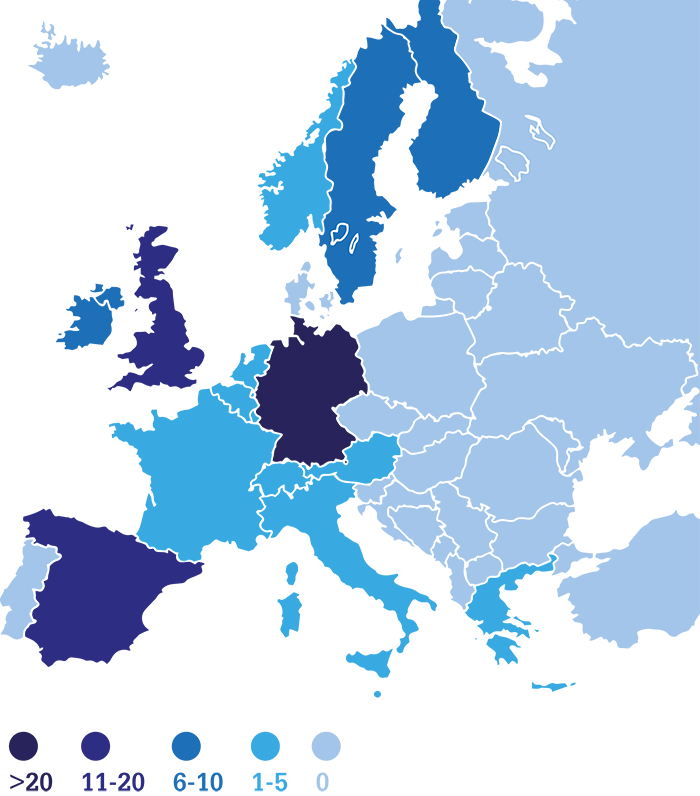
Our work on pharmaceuticals in wastewater started small. Spurred by warnings from both the media and scientific experts about the dangers of pharmaceuticals entering our water supplies, we initiated a student laboratory project to collect water samples from a nearby river, downstream of a wastewater treatment plant. We fully expected to find nothing – assuming any drugs would be removed during processing at the plant. Nevertheless, we conducted a few concentration steps using simple solid-phase extraction cartridges and ran some HPLC-ESI-QTOF-MS experiments. To our great surprise, tens of compounds were very easily detected. Both the number of drugs and the ease of detection struck us, so we decided to continue our research more systematically. An extensive literature search confirmed that pharmaceuticals in the aquatic environment are a global problem, but published studies on the phenomenon are patchy (Figure 1) – and the quest for solutions is ongoing. Many details remain unsolved. Which laboratory conditions can best model the elimination? Which conditions are most efficient? What solutions can be scaled up for wastewater treatment? Which solutions are economically feasible?
Degrading drugs and feminine fish
Pharmaceuticals used in human and veterinary medicine find their way into wastewater in a number of ways. If the active ingredient is not fully absorbed in the human or animal body after administration, the compound is excreted, enters the sewage system and eventually arrives at a wastewater plant. Drugs that are absorbed are excreted in the form of metabolites, and enter the same water circulation routes. Individual disposal via household waste and wastewater has little importance for the entry of drugs into the environment, but hospital wastewater exhibits a high concentration of pharmaceuticals (1)(2)(3). All wastewater streams are usually channeled to the same conventional sewage treatment plants. And though they have mechanical and biological purification stages, such plants are often unable to eliminate pharmaceutical drugs, specifically antibiotics, from wastewater circulation (4)(5)(6)(7). Wastewater plants may even concentrate the compounds as a consequence of the residence time within the plant. Usually, sewage treatment effluents are conducted into rivers, and the sewage sludge will be used as fertilizer in agriculture, allowing entry into groundwater. Furthermore, industrial wastewater from pharmaceutical plants can also lead to an increase of water pollution (8)(9). The number of studies on the occurrence of pharmaceutical compounds in surface and ground waters has tripled during the last decade. The typical concentrations of active compounds and their major metabolites are low, in the microgram/liter range (10)(11)(12)(13) – well below the therapeutically effective threshold for humans. However, the consequences in the aquatic environment are severe; for example, feminization of fish, loss of diversity, and even extinction of species. In fact, drug residues and their metabolites can be found in all compartments of the aquatic environment (14)(15)(16).The effect of drugs on the environment primarily depends on the degradability of the individual compounds. Biodegradation is influenced by many factors, such as pH value, temperature or further matrix properties, and is of great importance; the destruction of the synthetic chemical compound relies mainly on bacterial metabolism, degradation and eventual mineralization. However, photolysis, hydrolysis, and chemical reduction may all contribute to the compound’s fate. For humans, the consequences of continuous exposure to many drug substances are largely unknown. The concentrations of active ingredients of most pharmaceuticals in surface water, groundwater and drinking water are thought to be too low to cause toxic effects in humans. But the effects of long-term, low-dose administration of many drugs are not well (or easily) studied, so it’s hard to say with any certainty. Direct toxicity is not the only potential impact. One of the greatest problems facing human and veterinary medicine is the emergence of antimicrobial resistance (AMR). Primary antimicrobial resistance occurs frequently in nature; for example, the resistance of Pseudomonas aeruginosa to penicillin G. However, secondary resistance results from the contact of microorganisms with antibiotics. Bacteria can then share the genes for resistance via extrachromosomal genetic material passed on by conjugation. The resulting resistant bacteria may find their way into the environment and adversely affect aquatic and terrestrial organisms. Eventually, they may reach humans via the food chain. As the occurrence of antibiotic-resistant bacteria becomes increasingly frequent, our armory of effective antibiotics shrinks (17). Is massive entry of antibiotics into open waters via wastewater and effluents of wastewater treatment plants a contributing factor to antibiotic resistance? The jury is still out. Interestingly, urban wastewater has a similar number of resistant bacteria as hospital wastewaters with a high level of antibiotics (17). Even in urban wastewater with low concentrations of antibiotics, multiresistant E.coli, Acinetobacter, Enterobacteriaceae and Pseudomonas were detected. This finding could suggest that the emergence of resistant bacteria is actually caused by bacteria already rendered resistant by prior antibiotic treatment (17) rather than antibiotics in the environment, but is by no means definitive.
Stemming the tide
In our quest to reduce the entry of pharmaceuticals and their metabolites into the aquatic environment, we focus on the photo-induced degradation of active pharmaceutical ingredients. Using UVA and UVC irradiation in combination with a batch reactor, we have investigated the effect of pH, chemical additives and catalysts on degradation. By studying the structure of the phototransformation products and the velocity of the degradation, we can assess the efficiency of the conditions and catalysts chosen and, in doing so, discover the most effective combinations. We also investigate the chemical kinetics not only of the main compound, but also of its degradation products. We mostly rely on high-performance liquid chromatography (HPLC) coupled to high-resolution and/or higher-order mass spectrometry (MS). A linear ion trap and a quadrupole time-of-flight mass spectrometer equipped with electrospray ionization sources are our instruments of choice. Fragmentation techniques and accurate mass determination elucidate the structure of the degradants, while the time-of-flight analyzer ensures unbiased identification of all analytes in a water sample. We quantitate the results using the mass area, which is usually considered representative for the analyte concentration. Nevertheless, it is important to note that the ionization efficiency of an analyte is compound-specific and also dependent on the experimental conditions, particularly on the solvent composition at the retention time. We test the concentration–time (more precisely, the mass area–time) curves resulting from HPLC-MS analysis during an irradiation experiment against different kinetic models, to obtain the reaction order, degradation rate constant and corresponding half-lives of educts and products. The toxicity of degradants can be predicted on the grounds of their chemical structure and known structure–activity relationships, but we also conduct toxicity assays. Our ultimate goal is to ensure complete mineralization of a compound, so that no potential for environmental harm remains.A sea of data
A major challenge for the identification and quantitation of the pharmaceuticals is the complexity of the matrix of the different waters – coupled with the low concentrations. The large volume of analytical data involved renders interpretation particularly tedious, especially for conventional univariate data analysis. To this purpose, multivariate data analysis techniques have been thoroughly investigated and applied during the last decade (18)(19). Indeed, we rely heavily on multivariate data analysis and chemometrics to analyze the complex data obtained, using MatLab software and its applications, such as the PLS toolbox from Eigenvector Research (see Figure 2).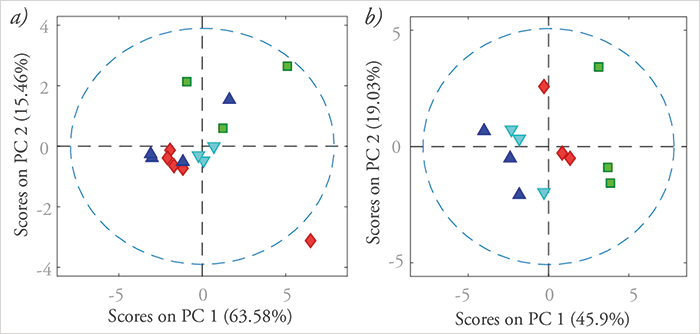
Multivariate data analysis methods applied in environmental sciences can be divided into two main groups: quantitation using regression methods, and qualification or identification using classification methods. The most common multivariate data analyses for classification purposes are principal component analysis (PCA), factor analysis (FA), cluster analysis (CA) and discriminant analysis (DA). Applications of chemometric methods in wastewater analysis include chemical mapping of antibiotics and identifying sources of entry and retention in the environment (20)(21). For quantification, multivariate linear regression (MLR), partial least squares (PLS), multivariate curve resolution (MCR) and parallel factor analysis (PARAFAC) are often used (18) (22), alone or in combination. The determination of pharmaceutical concentration in surface water was described in a recent illustrative study using PCA and CA (19)(23)(24). Studying the photo-induced degradation of pharmaceuticals under different conditions resulted in data with complex interdependencies, not only with respect to the kinetic parameters but also to compound descriptors, such as lipophilicity, logP, acidity, pKA, and others. Exemplar PCA results are presented in Figure 2. The left diagram (a) relates classes of antibiotics to their degradation behavior. Plot (b), on the right, relates the reaction conditions to the reaction rates. It quickly becomes obvious that harsher reaction conditions (for example, the presence of oxygen and hydrogen peroxide) foster faster degradation, as expected.
Breaking it down
To eliminate pharmaceuticals from wastewater, a so-called ‘fourth purification stage’ in wastewater treatment plants has been discussed for several years (25)(26) and the use of advanced oxidation processes (AOPs) and other degradation techniques has been explored. By using AOPs, oxidation of chemical compounds is achieved via a combination of UV irradiation and ozone or hydrogen peroxide (H2O2), or through ozone and hydrogen peroxide and catalysts. Whichever method is used, the result is an increased formation of hydroxyl radicals (OH•), which react very rapidly with almost all organic compounds because of their high oxidation potential. Alternatively, titanium dioxide (TiO2) can be used as a heterogeneous catalyst. Likewise, the photo-Fenton reaction creates a significant amount of hydroxyl radicals (27)(28)(29)(30)(31). The presence of H2O2 as an additive is known to be favorable for the acceleration of degradation (32)(33)(34). But when the concentration of H2O2 is too high, there is a risk of supersaturation. As shown in Table 1, we found that supersaturation was only observed for levofloxacin, azithromycin and sulfamethoxazole – all other active ingredients studied were degraded more rapidly with increasing concentrations of H2O2. Such detailed knowledge about the mechanisms and kinetics of degradation is essential if we are to achieve complete elimination of drugs from wastewaters.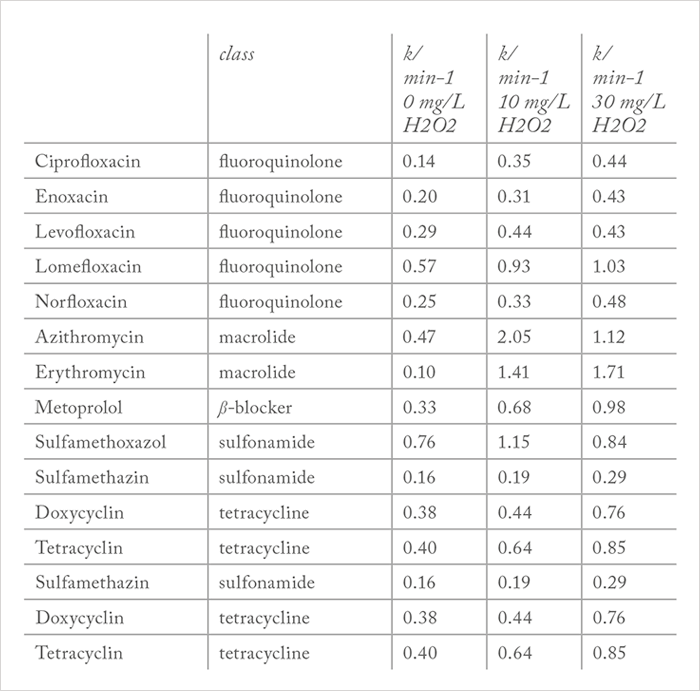
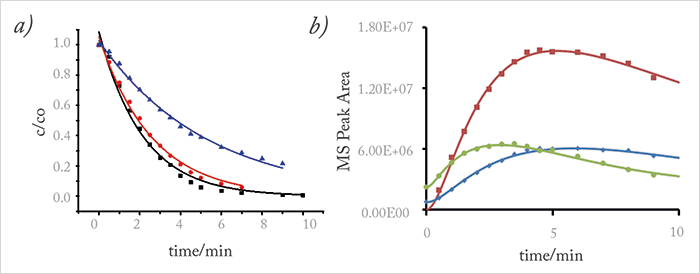
On UV-irradiation of drugs, phototransformation products are formed, which can be just as dangerous in environmental terms as the parent molecules. To predict whether a drug and its phototransformation products are significantly degraded, we must determine the kinetic rate constants. Most publications report first-order kinetics for the degradation of the main pharmaceutical compound (34), but not the chemical kinetics of the photo-induced transformation products. We set out to rectify this omission by determining both the degradation rates and the half-lives of phototransformation products. Figure 3 (a) shows examples of the c-t curves of azithromycin and their description according to a first-order reaction model. Different pH values lead to different degradation velocities as indicated by the reaction rate constants; for example, azithromycin degraded slower at pH 9 than at pH 3. The c-t curves of three identified phototransformation products are displayed in Figure 3 (b). These curves could result from follow-up (red, green, blue) and subsequent follow-up (red, green, blue) reactions. It’s clear that degradation products are still present when most of the drug has been eliminated. Hence, it will be important to understand their environmental toxicity or adverse effects.
All aboard
We need to address the issue from several angles. A chemical or photochemical purification step could be added in wastewater treatment plants, but also at individual sources of effluents, such as hospitals. Purification closer to the source would allow better elimination, since the drugs won’t have been diluted through the sewage system. However, economically feasible translation of purification methods into large-scale operations is still a future aspiration. Perhaps most urgently, we need a better understanding of the toxicity of degradation products. From a more strategic point of view, we must also consider how we might tackle the problem at its source; for example, by using more thoughtful prescription strategies for drugs such as antibiotics in human and veterinary medicine.
Protecting our water system from errant pharmaceuticals represents a significant challenge for science – and analytical chemistry has an essential role to play. Only with a much greater understanding can we decide what steps must be taken and how quickly; to not act is to turn a blind eye to long-term consequences for our home planet. Melanie Voigt, Anika Gold, Christina Blaesing, Timo Hoelscher and Martin Jaeger are researchers at Niederrhein University of Applied Sciences, Department of Chemistry, Instrumental Analytical Chemistry, Germany.
References
- KD Brown et al., Sci Total Environ, 366, 772–783 (2006). AY-C Lin, Y-T Tsai, Sci Total Environ, 407, 3793–3802 (2009). K Kümmerer, Chemosphere, 45, 957–969 (2001). F Baquero et al., Curr Opin Biotechnol, 19, 260–265 (2008). C Bouki, Ecotoxicol Environ Saf, 91, 1–9 (2013). I Michael et al., Water Res, 47, 957–995 (2013). GD Wright, Adv Drug Deliv Rev, 57, 1451–1470 (2005). M Saleem, J Res Sci, 18,18, 125–134 (2007). AM Deegan et al., Int J Environ Sci Technol, 8, 649–666 (2011). K Kümmerer, J Antimicrob Chemother, 52, 5–7 (2003). K Kümmerer, Chemosphere, 75, 417–434 (2009). D Fatta-Kassinos et al., Anal Bioanal Chem 399, 251–275 (2011). C Yan et al., Environ Pollut, 175, 22–29 (2013). FP Offa, K State, Appl Chem, 69, 22974–22999 (2014). J Quintana et al., Water Res, 39, 2654–2664 (2005). A Nikolaou et al., Anal Bioanal Chem, 387, 1225–1234 (2007). K Kümmerer, Chemosphere, 75, 435–441 (2009). M Otto, VCH Verlagsgesellschaft mbH (1997). C Stedmon, R Bro, Limnol Oceanogr Methods, 6, 572–579 (2008). E Papa et al., Environ Sci Technol, 41, 1653–1661 (2007). PR Kannel et al., Anal Chim Acta, 582, 390–399 (2007). W Kessler, Multivariate Datenanalyse (2013). doi:10.1002/9783527610037 S Mas et al., Talanta, 80, 1052–1067 (2010). H Jiang et al., Environ Sci Pollut Res, 20, 9075–9083 (2013). S Parsons, Advanced oxidation processes for water and wastewater treatment, IWA Publishing (2004). T Oppenländer. Photochemical Purification of Water and Air: Advanced Oxidation Processes (AOPs): Principles, Reaction Mechanisms, Reactor Concepts (Chemistry), WILEY-VCH, Verlag (2003). V Homem, L Santos, J Environ Manage, 92, 2304–2347 (2011). J Rivera-Utrilla et al., Chemosphere, 93, 1268–87 (2013). B Wols, CHM Hofman-Caris, Water Res, 46, 2815–2827 (2012). AL Boreen et al., Aquatic Sci, 65, 320–341 (2003). R Andreozzi et al., Catal Today, 53, 51–59 (1999). I Kim et al., Chemosphere 77, 518–525 (2009). B Wols et al., Water Res, 47, 5876–5888 (2013). F Yuan et al., Water Res, 43, 1766–1774 (2009).




