
It almost goes without saying that high quality analytical techniques are crucial in medical and clinical applications…
Medical research would be extremely limited – if not unfeasible – without access to high quality analytical techniques, including mass spectrometry. Most critical research questions being investigated in biomedical research can only be answered by probing details of molecular processes in biological samples, which requires high-performance analytical measurements. Medical research has also become increasingly translational, with many investigators going directly to clinical studies with complex patient samples and often skipping pre-clinical studies with more controlled and less complex animal models.
Analyzing human tissues from large and diverse cohorts of patients in multi-institutional clinical studies demands the use of high-quality (reproducible and robust) analytical measurements – otherwise it would be very challenging to derive new and significant knowledge with high rigor and depth of information. One key example that comes to my mind are the many studies where investigators are trying to understand the molecular processes related to a patient’s ability to respond to certain therapies, such as immunotherapies for cancer. These are very intricate questions that have many variables that could be related to the outcomes – meaning that a deep characterization of molecular patterns of disease is of the utmost importance.

Would you agree that lipidomics has gained significant ground over the last decade?
Absolutely! I love lipid analysis and have been a huge promoter of the value and depth of information that these incredible molecules carry in biomedical research. Lipids are beautiful molecules with a high diversity of both chemical structure and biological roles. Often, when we use the term “lipids” to a general audience their minds go directly to common dietary fats – but in the last decade or so, there has been rising awareness that lipids are key components to our cells’ structures while playing vital signaling roles.
My personal favorite class of lipids are cardiolipins. First, because their chemical structure is quite unique with four fatty acid chains – but also because these lipids are almost exclusively found in the mitochondrial membrane, and their abundance and structure have been significantly associated with metabolic and respiratory processes that have major implications in cancer. The ability to measure these molecules has been increasingly sought after, as more people are thinking about the correlation between mitochondrial metabolism and disease state.
I believe one major reason why more people are paying attention to lipids is because mass spec techniques are now sufficiently advanced to allow direct analysis and imaging of lipids from unmodified human samples. Furthermore, we now know that genomics and proteomics alone cannot entirely explain diseases and outcomes, which has led many investigators to value lipidomics and metabolomics as an essential part of their biomedical studies.
How might recent advances in lipidomics change the day-to-day work of diagnostic professionals?
I believe it is about time that we see some real changes in clinical diagnostics by incorporating lipids into clinical analysis and decision making. There is a substantial body of scientific and clinical work that supports the translational implementation of lipidomics into routine clinical testing. There are three advances that have enabled this implementation: i) the aforementioned mass spectrometry tools that allow rapid analysis of lipids directly from patient specimens, ii) the ability to measure lipids with high chemical specificity, and iii) data analytics and machine learning algorithms that allows us to convert lipidic information into clinically actionable data.
The technology is available and ready – what we need now is standardization of methods, validation of results, and the effort to pursue the regulatory steps to make this possible.
What’s your research mission?
I am extremely passionate about bringing mass spec to medical practice and enabling medical users with limited or no expertise in analytical chemistry to use mass spectrometry and its ability to gather incredible molecular information to make better decisions for their patients – especially patients with cancer.
And that means developing and implementing user-friendly mass spectrometry techniques that can rapidly measure the incredibly rich lipid and metabolic composition of human specimens. We are very passionate about using high-performance mass spectrometry to characterize patterns of lipids or specific lipid molecules that can be correlated to disease state, subtype, outcomes, and response to treatment. We use refined data analytics and biostatistical tools to translate that information to actionable outputs that can guide medical doctors in treating patients. We are, of course, also very interested in accessing lipid information from human tissues and correlating the mass spec data with data from other molecular assays (for example, proteins and genes) to better understand biological processes related to disease development, progression, and treatment outcomes.
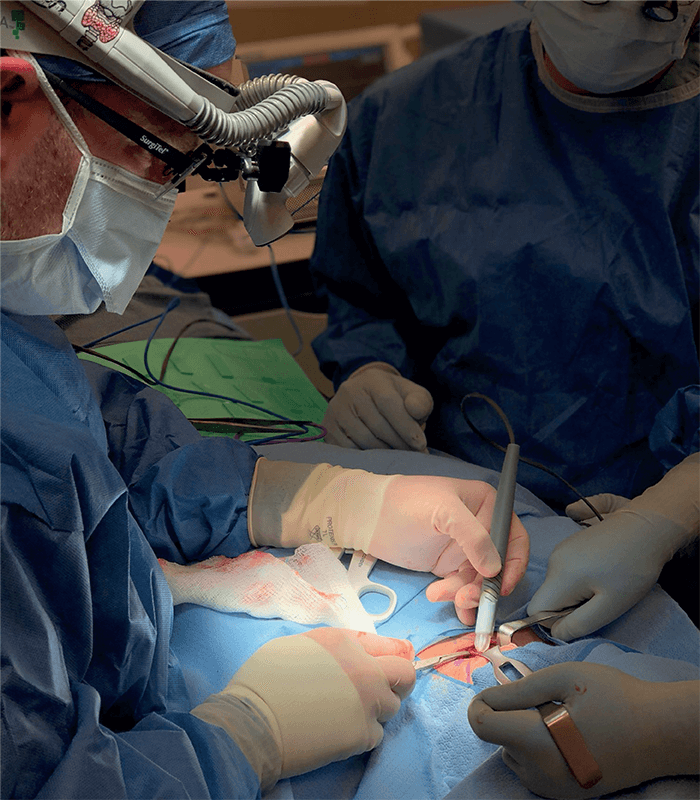
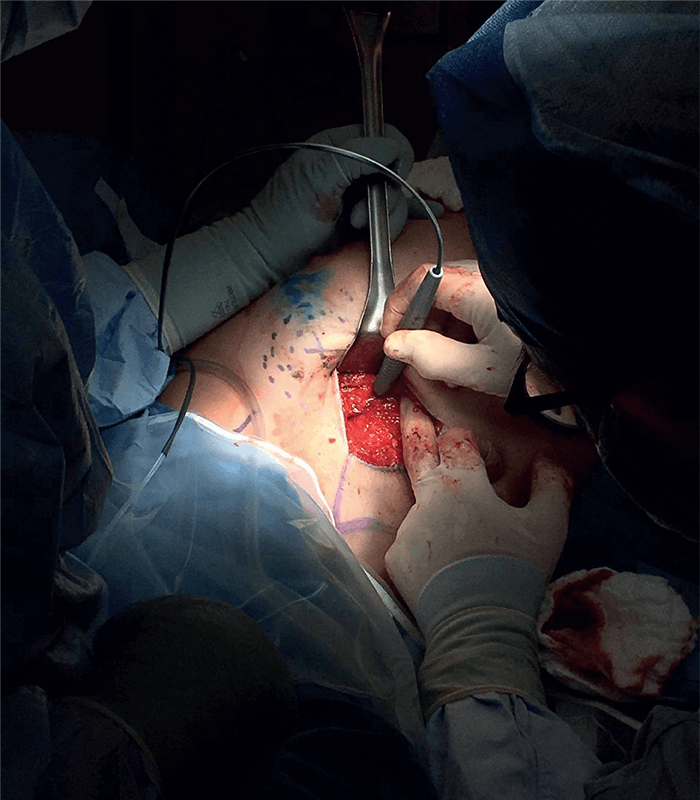
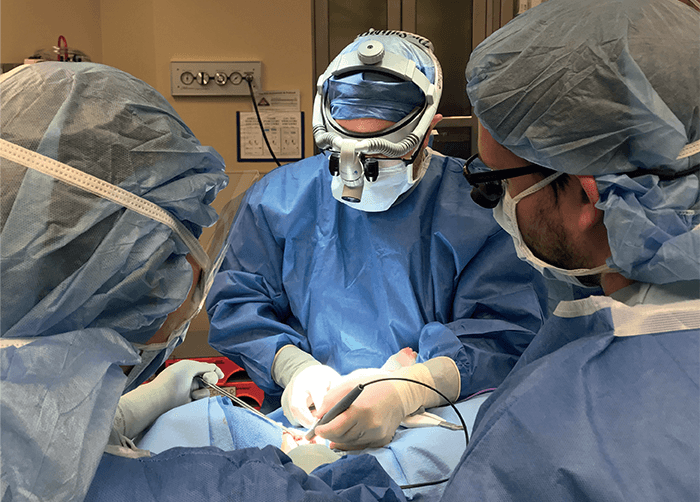
Could you please share some recent research highlights?
A major research effort in our lab involves several projects in which we are translating and testing the MasSpec Pen technology in clinical practice to help surgeons and pathologists identify and diagnose tissues during surgical procedures. The MasSpec Pen is a handheld device that enables gentle and rapid analysis of tissues in vivo or ex vivo. The device is easy to use and allows non-expert users to perform direct mass spec analysis of lipids and metabolites from tissues in seconds, with minimal training. The pen employs a very basic chemical principle for fast and efficient lipid analysis from tissues: it deposits a solvent droplet onto a tissue, which extracts lipids and metabolites, and then transfers the droplet containing the molecules to a mass spectrometer for immediate analysis. This molecular analysis is non-destructive – meaning that there is no damage to the tissue from the gentle lipid extraction, meaning that the tissue can still undergo standard clinical analysis. The non-destructiveness of the techniques has also allowed us to do repetitive tissue analysis in vivo, allowing molecular-based tissue identification prior to excision, which is truly transformational to clinical practice.
Over 20 surgeons have now used the MasSpec Pen in the operating room in ongoing clinical studies at MD Anderson Cancer Center and Baylor College of Medicine at the Texas Medical Center, US, across many diseases including breast, pancreas, thyroid, ovarian, and lung cancers. In a recent study that we published in PNAS (1), we used the MasSpec Pen to analyze 157 banked human tissues, including pancreatic ductal adenocarcinoma, pancreatic, and bile duct tissues – yielding rich molecular profiles characterized by high abundance of metabolites, fatty acids, and lipids. We then used the molecular data to generate classification models and achieved an overall 91.5 percent agreement with pathology for discriminating normal pancreas from cancer – including histologically complex samples with low tumor cell concentration evaluated within our test set. Key molecular predictors in the classifiers included lipids species, including glycerophosphoinositols. We then translated the MasSpec Pen to the operating room and predicted on in vivo and ex vivo data acquired during 18 pancreatic surgeries, and achieved 93.8 percent overall agreement with final postoperative pathology reports. Notably, when we integrated the banked tissue data with intraoperative data, the agreement improved agreement to 100 percent.
We also have many studies in which we are using desorption electrospray ionization mass spectrometry imaging (DESI-MS imaging) to better understand cancer progression and outcomes related to treatments. For example, we recently published a study (2) in which we used DESI-MS imaging to investigate alterations in lipid profiles in tissues related to anti PD-1 treatment. We identified specific lipid alterations associated with the degree of response to anti-PD-1 treatment – including a significant increase of long-chain polyunsaturated lipids within responsive tumors following anti-PD-1 therapy. Immunofluorescence imaging of tumor tissues also demonstrated that the altered long-chain polyunsaturated lipids associated with treatment response were localized to dense regions of tumor immune infiltrates. These results indicate that effective anti-PD-1 therapy modulates lipid metabolism in tumor immune infiltrates, and provide evidence that further investigations of the related immune-metabolic pathways may be useful for better understanding success and failure of anti-PD-1 therapy – which is really exciting.
You are evidently excited and optimistic about the potential impact of your research…
The research that my lab and other groups are pursuing in direct lipid analysis using innovative mass spectrometry technologies have enormous potential in guiding treatment decisions for patients. The molecular data acquired in clinical studies shows that we can provide a rapid assessment of lipid profiles to immediately inform disease state and stratify cancer – but we also know that the depth of this data provides new biological knowledge of the molecular processes that are altered in these tissues to identify novel targets for therapies.
In my lab, we are particularly excited about the MasSpec pen – and the innumerous applications for clinical practice in enabling rapid and non-destructive assessment of tissues, providing access to molecular data from living human tissues in a way that has been very limited before.

And is that what makes your research so rewarding?
It is really amazing to see how the lipid-based molecular information (acquired in seconds with mass spec techniques) can be used clinically to make a huge impact on treatment decisions for patients. It is also extremely rewarding to witness the excitement from medical professionals who gain access to a high-performance technology – such as mass spectrometry – through user-friendly systems that can help them improve their practice and how they treat their patients in a way that was not possible before.
In clinical practice, “molecular” studies are still most commonly associated with genetic or proteomic research, and I would love for doctors to understand that there is a lot more to “molecular diagnostics” than DNA sequencing, q-PCR of RNA, or assays for protein analysis. Lipids are incredible molecules that can also provide highly diagnostic molecular information – and they are easily measured with mass spectrometry techniques! Incorporating these technologies and molecules into clinical decision making should provide a real advantage in our ability to better diagnose and treat patients.
There is a pressing need for our scientific community to conduct rigorous studies that help validate and standardize methods across institutions and research protocols to truly show the impact that lipid analysis by mass spectrometry can have in the clinic. Rigor in data and statistical analysis is absolutely essential – but we also need larger studies with broad and diverse patient cohorts to show the clinical utility of our methods in improving patient care across the world.
References
- ME King et al., “Rapid diagnosis and tumor margin assessment during pancreatic cancer surgery with the MasSpec Pen technology,” Proceedings of the National Academy of Sciences, 118, 28 (2021). DOI: 10.1073/pnas.2104411118
- ME King et al., “Long-chain polyunsaturated lipids associated with responsiveness to anti-PD-1 therapy are colocalized with immune infiltrates in the tumor microenvironment,” Journal of Biological Chemistry, 299, 3 (2023). DOI: 10.1016/j.jbc.2023.102902




