Mark J. Perkins,1 Vaughan S. Langford2 1Anatune Limited, Cambridge, United Kingdom 2Syft Technologies Limited, New Zealand
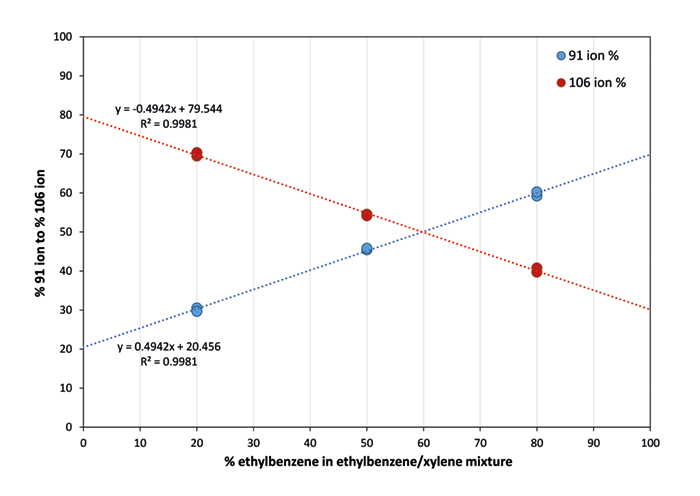
Resolution of ethylbenzene from the xylenes is important when regulators impose different emission or exposure limits, as is the case for occupational exposure limits in the European Union (time-weighted averages of 100 and 50 ppm, respectively). Direct mass spectrometry techniques traditionally struggle to distinguish these isomers, so measurement has been reported as a total concentration. In this application note we apply the SIFT-MS technique to achieve direct, real-time speciation of the xylenes from ethylbenzene. A Syft Technologies Voice200ultra SIFT-MS instrument – which applies multiple, rapidly switchable reagent ions with different ionization properties (see syft.com/SIFT-MS) – was used in this study. Air was sampled continuously through the instrument’s high-performance inlet at approximately 25 sccm. The total ethylbenzene + xylenes concentration was measured using the NO+ reagent ion. The O2+ reagent ion reacts with ethylbenzene and the xylenes to form the same product ions (m/z 91 and 106), but in almost inverse ratios. Separation of the isomers is achieved through calibration of the 91 to 106 ratios using several ethylbenzene/xylene mixtures at two nominal concentrations (150 ppbv and 10 ppmv). Calibration results are summarized in Figure 1.

Figure 1. Calibration of relative abundances for the m/z 91 and 106 product ions formed when O2+ reacts with different mixtures of ethylbenzene and the xylenes. Figure 2. Separation of ethylbenzene and xylenes using O2+ with the total concentration obtained using NO+ superimposed.
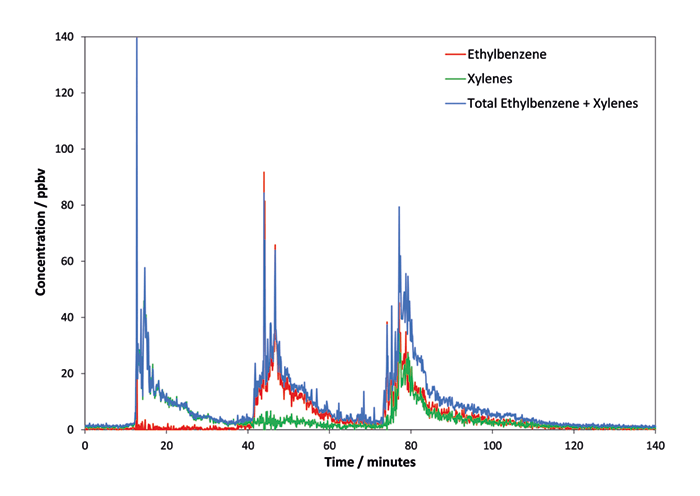
The success of this approach is illustrated from experiments conducted in the relatively complex air matrix of the Anatune Limited laboratory (typical concentrations in the 10s to 100s of ppbv). Data were acquired with a temporal resolution of just over three seconds. A small volume of each of m-xylene, ethylbenzene, and a mixture of the two was introduced into lab air from 20 mL headspace vials, which were shaken and uncapped for a few minutes. Laboratory windows were opened to ventilate the room between samples. Figure 2 shows the total concentration measured using the NO+ reagent ion, plus speciated ethylbenzene and xylenes concentrations obtained using O2+. It is clear that the first exposure was xylenes, followed by ethylbenzene and then a 50/50 mixture of the two. Excellent speciation of ethylbenzene from the xylenes in real time can be achieved at very low ppb levels by using the SIFT-MS O2+ reagent ion. This approach can be applied to both real-time and high-throughput applications, providing benefits in testing laboratories and continuous monitoring applications. For the full application note, visit syft.com/ethylbenzene-and-xylenes.







