In other areas the converse is true. Progress is too often hampered by our inability to gather the information needed to make advances. A topical example of this challenge is seen in the pharmaceutical industry where deformulation - the unpicking and rationalization of the performance of a reference labelled drug product - is an essential precursor to the development of a generic equivalent. The FDA has publicly acknowledged the difficulties in replicating complex drugs and has called for advances in analytical instrumentation to help.1
Applying analytical strategies that are well matched to informational requirements is essential for cost-efficient and timely progress in any field of scientific endeavour. When faced with the need to ‘dig deeper’ it’s easy to overlook the potential contribution of traditional methods, and instead to look to completely new solutions. However, today’s innovations are transforming the informational productivity of some of our workhorse techniques and have the enormous benefit of being rooted in tried and trusted technology. In this short series of articles I’ll be looking at important advances in three material characterization techniques – laser diffraction particle sizing, automated analytical imaging and gel permeation/size exclusion chromatography - that exemplify this trend. What these developments have in common is that they all substantially enhance the value of the analytical systems to reveal new levels of information about the materials under examination. The established technique: Laser diffraction particle size analysis Laser diffraction is one of the most widely used particle sizing techniques in industry, from R&D, through manufacture and into QC. Fast, non-destructive and highly automated it is a technique that is equally at home in the laboratory or out on the process, in the form of on- or in-line instrumentation. Over the past decade, new designs have refined laser diffraction instrumentation to the point where they are highly accurate and operate with ‘push button’ functionality. The focus of most laser diffraction measurements is to determine the size of primary particles rather than the size of any agglomerates that may be present in the sample (these latter tend to be less relevant to product performance). This makes it important to ensure that the sample is delivered to the measurement zone in an appropriately dispersed state. Developing a robust dispersion method is a pre-requisite to moving laser diffraction into routine use and one of the few remaining ‘expert tasks’ associated with the technique. Being able to securely judge the extent and success of a dispersion process is critically important. The innovation: The addition of imaging Advances in camera technology and processing power have transformed imaging technology over the last decade or so and the latest innovations for laser diffraction technology take full advantage of this. In-line imaging accessories for laser diffraction analyzers have recently become commercially available, and at an attractive performance/price point. These accessories provide real-time particle size and shape data during liquid sample dispersion. Monitoring how particle size and shape change over the course of the dispersion enables users to quickly identify when complete dispersion is achieved, or indeed if a proposed or applied method is failing to completely disperse the sample ahead of measurement. This makes it far easier and faster to develop a robust wet measurement method but of equal importance is that it provides some substantial insight in cases where the particle size data may be open to various interpretations. The reveal: particle agglomeration or crystal growth? Particle sizing is often used during long-term stability testing to determine whether a product changes over time. This is particularly important for pharmaceuticals, where the product shelf life is often expected to exceed two years and where any changes have the potential to impact clinical efficacy. The particle size of an indigestion remedy stored for several months time was measured using a laser diffraction analyzer with inline particle imaging accessory (Mastersizer 3000/Hydro Sight, Malvern Instruments). Measurements of the particle size distribution of the sample prior to dispersion indicated a bimodal distribution, which after dispersion using sonication became a narrow single-mode distribution centered on a relatively fine particle size. The obvious, and often correct, interpretation of this pattern of results is that loosely bound agglomerates, formed during storage, have been dispersed by wet dispersion. However, imaging reveals in this case that something different has occurred (see Figure 1). The larger-sized particle population present before the application of the liquid dispersion process is made up of a relatively small number of needle-shaped particles. These are highly unlikely to be agglomerates, because of their shape. Rather, the results suggest that under certain storage conditions particles present in the suspension may grow, perhaps as a result of other components precipitating out of solution. Dispersion using sonication breaks up these fragile particles. Here then, imaging proves that the usual explanation of the results obtained during sample dispersion does not apply. Instead it reveals that the large particles detected using laser diffraction are potentially problematic primary particles. This fact would likely have been missed without the imaging data, a clear demonstration of the potential value of using imaging and laser diffraction in tandem.
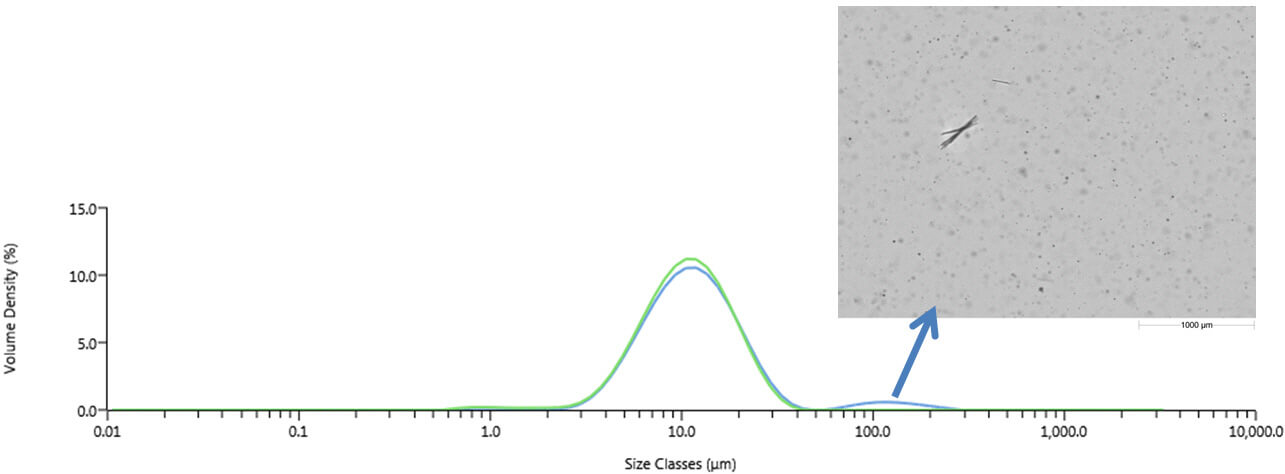
Figure 1: Laser diffraction results obtained before (blue) and after (green) sonication of the sample. Images taken prior to sonication clearly show large needled-shaped particles within the sample, suggesting that it is particle growth/precipitation that produces a bimodal distribution in the indigestion remedy, not agglomeration. Reference 1. FDA, “Critical Path Opportunities for Generic Drugs May 2007”, http://www.fda.gov/oc/initiatives/criticalpath/reports/generic.html.
Malvern provides the materials and biophysical characterization technology and expertise that enables scientists and engineers to investigate, understand and control the properties of dispersed systems. These systems range from proteins and polymers in solution, particle and nanoparticle suspensions and emulsions, through to sprays and aerosols, industrial bulk powders and high concentration slurries. Used at all stages of research, development and manufacturing, Malvern’s instruments provide critical information that helps accelerate research and product development, enhance and maintain product quality and optimize process efficiency. Our products reflect Malvern’s drive to exploit the latest technological innovations. They are used by both industry and academia, in sectors ranging from pharmaceuticals and biopharmaceuticals to bulk chemicals, cement, plastics and polymers, energy and the environment. Malvern systems are used to measure particle size, particle shape, zeta potential, protein charge, molecular weight, mass, size and conformation, rheological properties and for chemical identification, advancing the understanding of dispersed systems across many different industries and applications. www.malvern.com Material relationships http://www.malvern.com/en/





