
Two years ago, I built up the courage to travel to Amsterdam to convince Ron Heeren that he and his team needed to move to Maastricht, build a world-class MS imaging (MSI) institute, and directly embed it in a clinical environment. Such an institute, I believed, could bridge the gap that traditionally exists between the development of new technology and its clinical application. Indeed, the project’s big ambition is to fully exploit the integration of new technological imaging developments within the clinical environment. In the process of developing the idea for the Maastricht MultiModal Molecular Imaging Institute (M4I), Frans Ramaekers (scientific director of the MUMC research school GROW) was also able to attract Peter Peters (now co-director of M4I). Although Peter had a great offer on the table to move from NKI to Delft, the integrative nature of M4I convinced him to settle in Maastricht. Today, my role in the project is as initiator and clinical bridge/liaison.
The primary value of M4I really lies in the investment in human capital (rather than simply MSI hardware). Ron’s team is at the forefront of the development of MSI equipment (fundamentals, technology) and application (desorption, detection). But we also needed buy in from specialists in instrument development, physicists, chemists, medical specialists, pathologists and translational scientists. Bringing MSI techniques into clinical practice requires rapid standardized measurements. Up until this point, technical development typically focused on discovery, so we needed a different slant. The multimodal approach appears to easily attract the talent we need from various research fields, but the key to our success will be based on the willingness of all team members to have an open mind to each other’s field, specific scientific language and modus operandi. To optimize collaboration, we decided to mix the research teams across all research offices and to have common research meetings and seminars. That said, the clinical research culture is very different from the research culture of chemists and physicists. According to the science philosopher Thomas Kuhn, the two sides may have difficulty learning each other’s language and adapting to it. But hopefully this cultural border will create just enough research tension to result in Kuhn’s predicted “scientific revolution.”
Mass spectrometry imaging provides insight into the molecular basis of a clinical problem. Consequently, the nature of a problem can be defined more accurately – and the treatment adjusted accordingly. It becomes even more powerful when it is merged with other omics data, such as genomics and transcriptomics. Clustering objective patient information provides more insight in diagnosis and therapeutic success (response) – and it is independent of personal interpretation or insight (the various ‘schools’ of teaching). It can also help ensure that patients receive optimal treatment or, perhaps even more importantly, prevent the use of unsuccessful or harmful treatments; for example, chemotherapy being given to ‘non-responders’.
As a surgeon, it is of the utmost importance to know the nature of the tumor you are about to operate upon. However, it is sometimes still difficult to obtain the correct diagnosis using standard pathological screening. Mass spectrometry imaging may give us the opportunity to gain a more specific and objective diagnosis ahead of surgery.
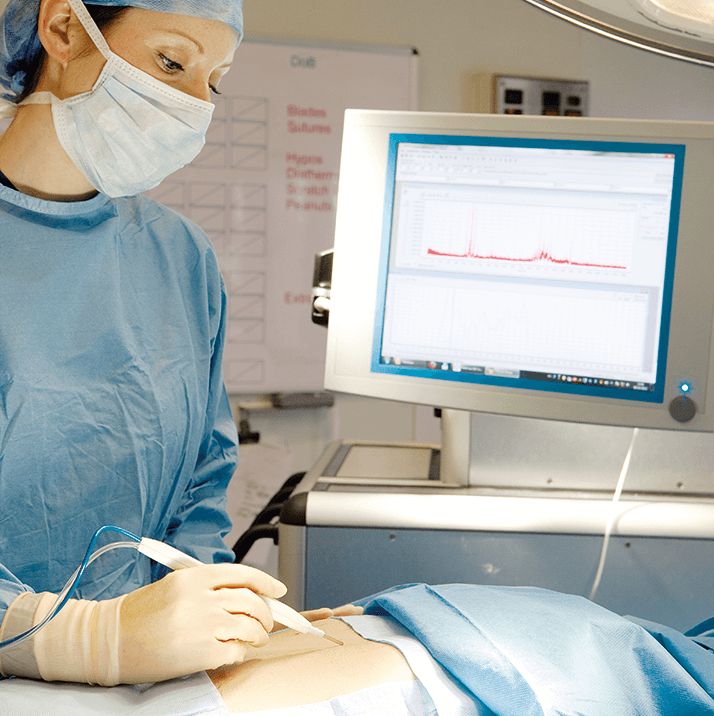
The research group of the Department of Surgery actually has a strong history of translational (metabolic) research. Specifically, we are strong in developing of specific human (patient) models to answer research questions, which allows us to move away from experimental animal models and therefore avoid the (patho-)physiological differences between species. It is my belief that most real changes are driven by technological (and that is to say analytical) advances. The close collaboration between hardcore scientists all focusing on the development of new tools that help guide treatment and predict (and evaluate) treatment success makes this initiative fascinating. There are also exciting developments in real-time diagnostics; we hope to implement MSI into the operating room were it could provide on-the-spot information on the tissue the surgeon resects. Such techniques could potentially avoid incomplete tumor resection by giving detailed molecular information about the cut-section, allowing surgeons to continue operating if necessary. And working with surgeon scientists allows Ron and Peter’s team to obtain optimally prepared human samples for imaging research. I think the biggest challenges we face are the standardization of analytical techniques and the building of metabolic profile libraries of diseases. In this latter endeavor, access to to the right patient samples is essential. No doubt, the introduction of new techniques into clinical practice requires both an acceptance of its potential and a change to the current workflow. I believe that we can only succeed by collaborating closely with our colleagues in the field. Our objectives will take some time to realize, but the first Horizon 2020 grant proposal has already been submitted.




