Robert Kennedy is the Willard Professor of Chemistry and Professor of Pharmacology at the University of Michigan. His research interests are analytical chemistry and its application to neuroscience, endocrinology, and biotechnology. His group developed instrumentation that couples sampling probes to capillary electrophoresis, capillary chromatography, mass spectrometry, and microfluidic assays for monitoring neurotransmitters in vivo. These methods have been used for studying changes in neurotransmitter concentrations associated with behavior and disease. Kennedy is Associate Editor of Analytical Chemistry and Director of the Microfluidics in Biomedical Sciences Training Program at Michigan.

Juan G. Santiago is Professor and Chair of the Thermosciences Group of Mechanical Engineering at Stanford University. His research includes the development of microsystems for on-chip chemical analysis, drug delivery, and sample preparation methods. Applications of this work include genetic analysis, drug discovery, and environmental monitoring. He is a Fellow of the American Physical Society, Associate Editor of the journal Lab on a Chip, and director of the Stanford Microfluidics Laboratory. Santiago has graduated 20 PhD students and advised nine postdoctoral researchers; 12 of these are now professors at major universities.

Albert van den Berg is professor and chair of the BIOS Lab-on-a-chip group at the University of Twente, Enschede, The Netherlands. His current research focuses on microanalysis systems and nanosensors, nanofluidics and single cells and tissues on chips. Applications in personalized health care, drug development and development of sustainable (nano)technologies are of particular interest to him. Van Den Berg has co-authored 250 papers, holds 10 patents, has participated in six spin-off companies, and is also Associate Editor of the journal Lab on a Chip. In 2011, he became a board member of the Royal Dutch Academy of Sciences (KNAW).

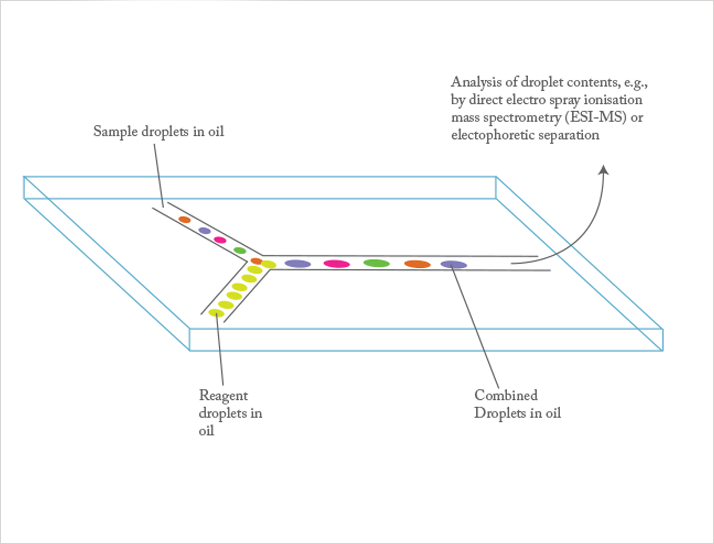
Robert: I have been very intrigued by the use of nanoliter and smaller droplets as tiny reaction and assay vessels within microfluidic systems. This is important because although microfluidic systems allow small sample consumption, it has not been trivial to introduce small quantities or handle discrete samples easily. Droplet methods solve this problem.

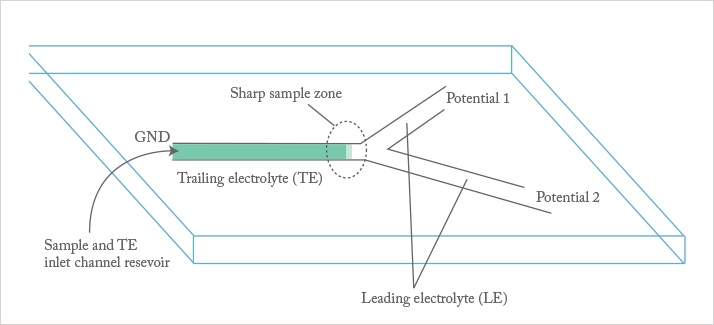
Juan: My interest in the last four years has focused around an electrokinetic technique known as isotachophoresis (ITP). ITP is decades old but I believe that it holds great potential for new functionalities and new assays. My work has concentrated on applying ITP to the grand challenge of automated and efficient sample preparation, and to the challenging problem of accelerating reactions involving macromolecules like RNA or proteins.

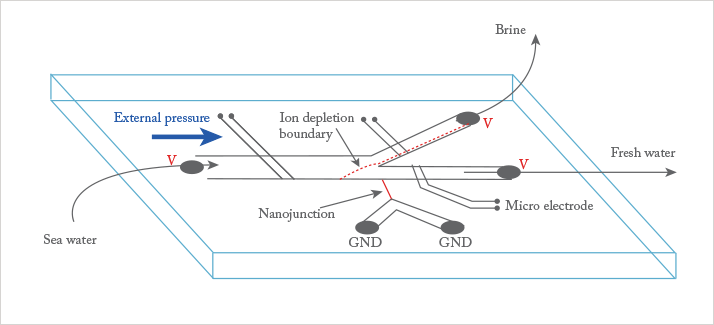
Albert: I am enthusiastic about the biomolecular preconcentration work using ion concentration polarization (ICP) originating from Jay Han’s group (1). Although not fully understood, the method gives a tremendous preconcentration effect leading to much better detection limits. It is interesting because it is relatively simple to do, makes use of double layer and electrokinetic effects, has complex theory, and a variety of applications.

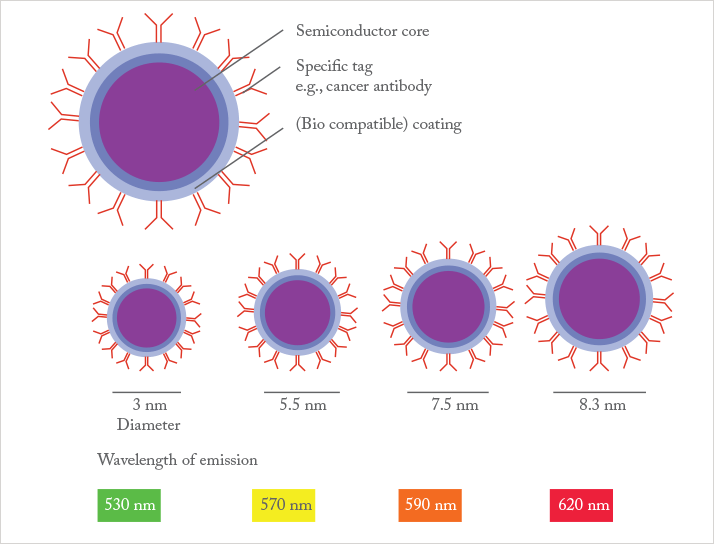
Robert: This is a tough one. I have to say that the initial work on microchip CE by Harrison and Manz (2) sparked a tremendous flood of research that has brought us to a high level of technical sophistication for microfluidic devices. Early work on measuring electrical effects of molecules through nanopores has also generated a lot of interest in using nanoscale devices for analysis. Finally, using nanoparticles as simple labels in assays, for example in work by Chad Mirkin (3), and early work on Quantum dots by Alvistatos and Nie (4) are both very important for nanoscale techniques.

Juan: I believe the greatest advances have been seen in microscale, not nanoscale, devices and techniques. Significant breakthroughs in the last decade include the advent, commercialization, and growth of droplet-based PCR systems (digital PCR) and on-chip electrophoresis devices for the size analysis of RNA and DNA, such as Agilent’s Bioanalyzer system. Furthermore, microfluidics continues to plays an important supporting role in a wider range of applications, including deep sequencing and proteomics.

Albert: I will mention two. First, the new sequencing approaches are exciting. On the one hand there is the work now commercialized by Ion Torrent, where sequencing by synthesis is accomplished using a “good old” integrated pH sensor (ion-sensitive field-effect transistor (ISFET)) array. On the other hand, a lot of progress has been made with translocation and sequencing of DNA though nanopores. In both cases, there is a strong link with micro- and nanofabrication and nanoscale analytics. Second, there is a lot of activity using new materials, such as carbon nanotubes, graphene and nanoparticles, that improve surface area, create tiny nanopores or enhance optical detection techniques.

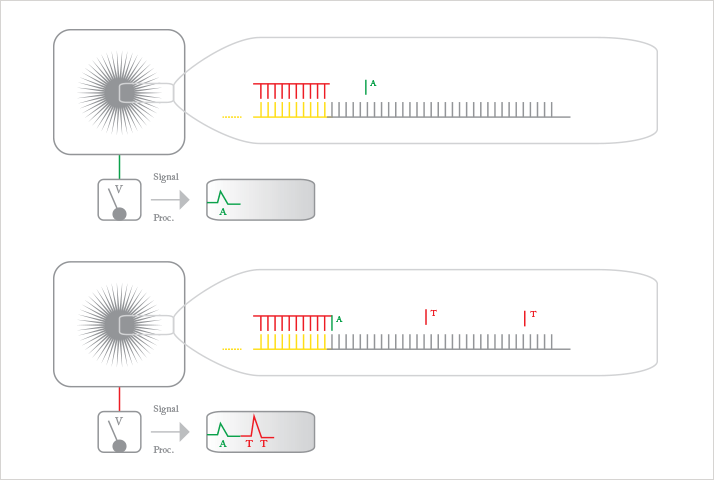
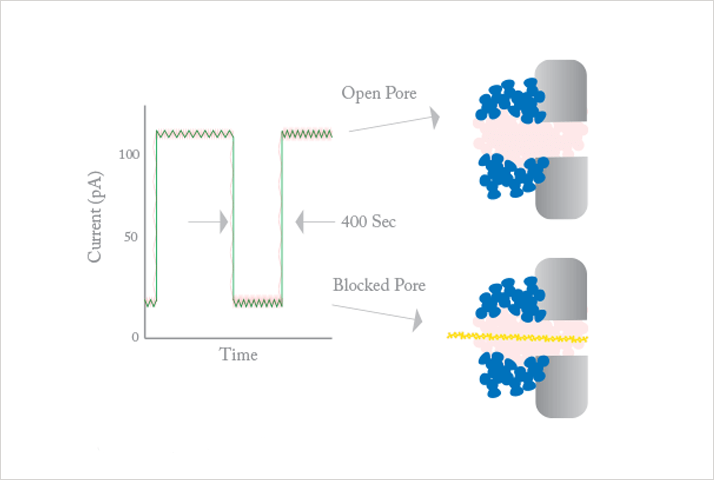
Robert: There is very little infrastructure around single droplet or microfluidic systems. Everything you want to miniaturize or do on a small scale must be developed or mastered from another laboratory. This makes the whole research process slow-going at times. For example, we wanted to be able to remove aliquots from nanoliter samples, so we had to build an entire project around how best to do that. Probably the biggest challenge for the future is finding the "killer application". Nanoscale analysis can make some things better, but is it worth giving up the tried and tested?!
I believe that screening applications very well could be a major area. Also, clinical assays, for example, isolating cells for diagnosis and then analysis, will probably be another big area.

Juan: I believe the highest rate of uptake in the next five years will continue to be from researchers in the life sciences – as has been the case in the last decade. Clinical applications offer great potential and market volume, but clinical diagnostics typically require immense investments, significant development times, and risk. These are starting to arrive and poised to grow, but it will take money and time.

Albert: I would say that genomics – everything dealing with DNA sequencing – is profiting most from nanoscale technology. I am convinced that a $1000, or even a $100, sequencer will become available within the next 10 years. Besides that, I also strongly believe that distributed, point-of-care diagnostics will be realized by the use of nanoscale technologies. Finally, of course, (medical) imaging is one of the largest driving forces behind many nanoparticle research efforts.

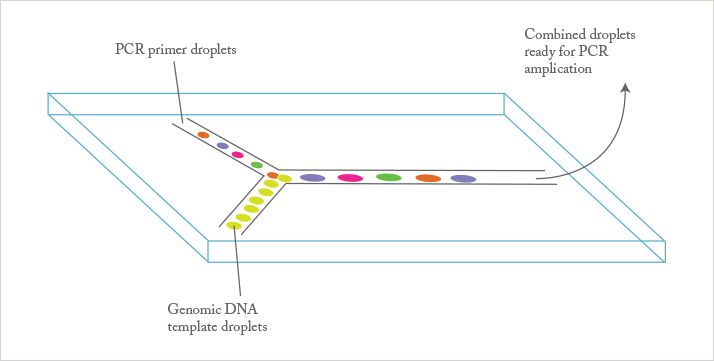
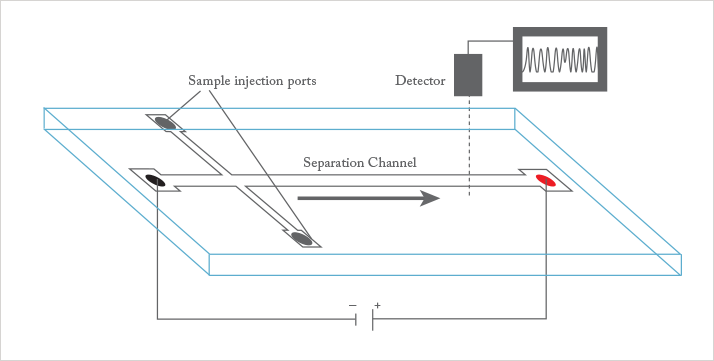
Juan: Micron-scale microfluidic devices offer parallelization, integration, and control of multiple processes in a single device. In my work, we are combining electrokinetic sample preparation (from complex samples including blood, urine, and cell cultures) with assays based on enhanced reaction kinetics between target molecules and a variety of synthetic, fluorescently-labelled probes.

Albert: We use micro- and nanofabrication techniques with our MESA+ Nanolab. This allows, for instance, extreme control of microstructures to minimize peak broadening in classical separation systems. It also allows fabrication of ultraprecise arrays of nanopyramids and nanogaps leading to homogeneous, highly sensitive surfaces for surface-enhanced Raman scattering (SERS). Another aspect is economy of (small) scale: cheap microscale complementary metal–oxide–semiconductor (CMOS) transistors can be used for DNA sequencing. But nanostructuring also allows exact control of nanogaps between electrodes, enabling redox cycling sensing with extremely high sensitivity. Because of size similarity, nanochannels and nanopores are very interesting structures to study biomolecular interactions and sequence DNA.

Robert: We and other groups have developed ways of doing high-throughput mass spectrometry on single droplets or large arrays of droplets. We have explored using this for screening, but it may also play a great role in enabling protein evolution and single cell analysis.

Juan: In essence, we have developed methods for rapid sample preparation, mixing, reaction, and separation. These are basic process steps for a wide variety of assays across genomics and proteomics. I envisage, for example, that ITP sample preparation could be a front-end for a wide range of techniques including digital PCR and sequencing.

Albert: We have recently started working with the microdroplet platform. This is a powerful tool because it creates picoliter confinements/environments that are useful for single cell analysis, single catalytic particle study, or single enzyme study. Furthermore, it is high-throughput; typically, 1000 droplets per second can be handled, as illustrated in pioneering work by Ismagilov (5), Weitz (6), Stone (7), Whitesides (8), Quake (9), Huck (10), etc. We believe microfabricated devices with integrated sensing will lead to a variety of disposable diagnostic devices for point-of-care applications.

Juan: One challenge is the increased importance of the surface chemistry of a device, and its effects on reproducibility and robustness of the assay. In many assays, we have tried to combat this issue by suppressing electroosmotic flow and non-specific binding to surfaces, while using electric fields and pressure-driven flows to control the motion of analytes in the bulk liquid.

Albert: Stable manufacturing is an important issue, as is making the connection between nanoscale and real life macrodimensions. And, as we become more prone to using top-down nanofabrication, maintaining the expensive infrastructure is a continuous and major concern. Apart from that, going to nanoscale also poses the question of how to bring analytes from, for example, millilitre samples to a nanometre-sized detector; sample preparation becomes the major challenge.

Robert: 5-10: I could see high throughput screening done at very low cost, based on utilization of nanoscale analysis, perhaps even bench-top screening done by individual investigators. In addition, routine single-cell isolation and analysis including proteomic and metabolomic analysis.
50: single cell dissection and surgery to remove select components for analysis.

5: improved chromatography; massively electrically readable DNA/protein arrays.
10: routine nanopore DNA sequencing.
50: continuous personal health monitoring for early diagnosis/preventive medicine.

Robert Kennedy: kennedygroup.lsa.umich.edu

Juan G. Santiago: microfluidics.stanford.edu/

Albert van den Berg: www.utwente.nl/ewi/bios/

References
- www.rle.mit.edu/micronano D. J. Harrison et al., “Capillary Electrophoresis and Sample Injection Systems Integrated on a Planar Glass Chip,” Analytical Chemistry 64, 1928-1932 (1992). sites.weinberg.northwestern.edu/mirkin-group www.cchem.berkeley.edu/pagrp ismagilovlab.caltech.edu weitzlab.seas.harvard.edu/research/microfluidics.html www.princeton.edu/~stonelab/ gmwgroup.harvard.edu thebigone.stanford.edu www.ru.nl/physicalorganicchemistry




