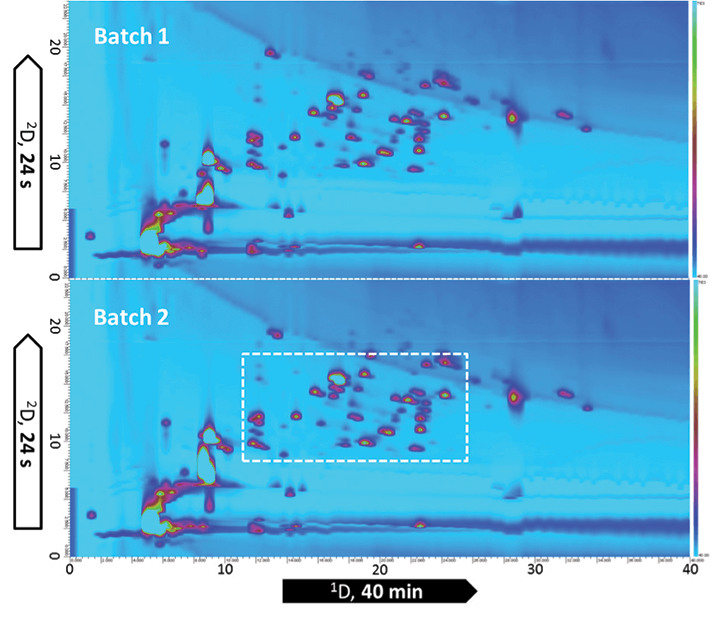
In recent years, the top ten pharmaceuticals sales list has been extensively populated with protein therapeutics, such as monoclonal antibodies and recombinant proteins, that are used to treat various life-threatening diseases, including cancer and autoimmune diseases. A number of these blockbuster biopharmaceuticals are coming off patent in the near future, a fact that has resulted in an explosion of activity in the biosimilar or “biogeneric” market. Irrespective of whether a company is developing innovative biopharmaceuticals or copycat biosimilars, a detailed characterization must be performed. Indeed, product characteristics need to be very closely monitored prior to clinical or commercial release. With complexity far exceeding that of small molecule drugs, the characterization of protein biopharmaceuticals represents significant analytical challenges, typically involving a wide range of analytical techniques and methodologies (1). Peptide mapping is a commonly used characterization methodology as it provides great detail of the molecule under investigation. Let’s take a monoclonal antibody of 150 kDa as an example; a trypsin digestion will generate over 100 peptides all with varying physicochemical properties over a wide dynamic concentration range. The complexity associated with these digests demands the very best in terms of separation power. Compared with one-dimensional separations (1D-LC), two-dimensional LC – and especially comprehensive 2D-LC (LC × LC) – drastically increases peak capacity as long as the two dimensions are orthogonal and the separation obtained in the first dimension is maintained upon transfer to the second dimension. Orthogonal combinations for 2D-LC based peptide mapping include strong cation exchange and reversed phase LC (SCX × RPLC); hydrophilic interaction chromatography and reversed-phase LC (HILIC × RPLC); and reversed-phase LC and reversed-phase LC (RPLC × RPLC) with different pH levels in the two dimensions (2-4). The highest orthogonality is obtained with SCX × RPLC and HILIC × RPLC, because the separation mechanisms are completely different. However, RPLC × RPLC is particularly interesting; excellent solvent compatibility in both dimensions makes it the most rugged, but it still offers very high peak capacity (driven by high plate numbers in the individual dimensions) and great orthogonality, which is due to the zwitterionic nature of peptides, such that major selectivity differences are achieved when performing separations on RPLC at pH extremes. Figure 1 shows the 2D-LC tryptic peptide maps of two production batches of the monoclonal antibody trastuzumab using RPLC × RPLC. Trastuzumab has been marketed as Herceptin since 1998, and is still being used in the treatment of HER 2 positive breast cancer. The peptide map provides a wealth of information that allows identity and purity to be assessed. Trastuzumab’s identity is spread over 62 tryptic peptides (20 light chain and 42 heavy chain) of which the majority is baseline resolved. Several of these tryptic peptides contain amino acids that are prone to modifications, such as deamidation, isomerization, oxidation, and so on. Such product related impurities can have an influence on both the safety and efficacy of the product and need to be closely monitored.
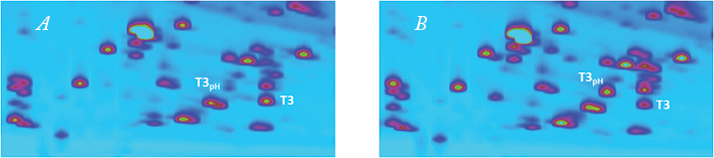
The benefits of 2D-LC technology with respect to such modifications are highlighted in Figure 2, which shows a zoomed view of the 2D-LC peptide map of a non-stressed and pH stressed originator. A deamidation event is substantially increased upon stressing trastuzumab at pH 9 for three days. This modification, which occurs on an asparagine located within the third tryptic peptide of the light chain, is already observed at around 10 percent in the non-stressed sample. Peak identity was obtained using MS and modification sites were revealed by MS/MS.
The precision associated with the RPLC × RPLC methodology combined with UV-detection (retention time RSD < 0.2% and area RSD < 5% for n=5) makes this very a powerful approach to demonstrate comparability between production batches, as demonstrated in Figure 1, and between originator biopharmaceuticals and biosimilars.
So, the big question is: where in the biopharmaceutical development pipeline do we see the true benefit of 2D-LC? It’s fair to say that in early stage development, one dimensional-LC-MS is certainly an option; RPLC columns that provide peak capacities well in excess of 500 are widely available nowadays – when used in combination with high-resolution mass spectrometry, a powerful characterization engine emerges. However, as we move along the development pipeline and enter the clinical or commercial release phases, MS preferably has to be replaced by UV detection and the enhanced resolution offered by 2D-LC comes into its own. Certainly, the appearance of commercial instrumentation represents a major step towards more widespread use of 2D-LC in biopharmaceutical analysis.
Koen Sandra is R&D Director, Gerd Vanhoenacker is LC Product Specialist/Manager, and Pat Sandra is Founder and President, all at the Research Institute for Chromatography (RIC), Kortrijk, Belgium.
References
- K. Sandra, I. Vandenheede, P. Sandra, J. Chromatogr. A, 1335, 81-103 (2014). G. Vanhoenacker, K. Sandra, I. Vandenheede, F. David, P. Sandra, U. Huber, E. Naegele, Agilent Technologies, Appication Note 5991-2880EN (2013). G. Vanhoenacker, I. Vandenheede, F. David, P. Sandra, K. Sandra, Anal. Bioanal. Chem, Special Issue “Multidimensional Techniques”, in preparation. K. Sandra, M. Moshir, F. D’hondt, R. Tuytten, K. Verleysen, K. Kas, I. Francois, P. Sandra, J. Chromatogr. B, 877, 1019-1039 (2009).