Researchers have synthesized a new class of chiral molecules that remain locked in a single enantiomeric form for tens of thousands of years, even at room temperature. The unprecedented stability of these compounds – whose central carbon atoms are bonded only to nitrogen and oxygen – marks a significant step forward in stereoselective molecular design and the control of molecular asymmetry.
The study, published in the Journal of the American Chemical Society, centers on carbon-based stereocenters that defy conventional bonding patterns. Rather than the typical carbon–carbon attachments found in chiral molecules, the new scaffolds feature quaternary carbon atoms bound entirely to heteroatoms. This arrangement had not previously been isolated in a stable form.
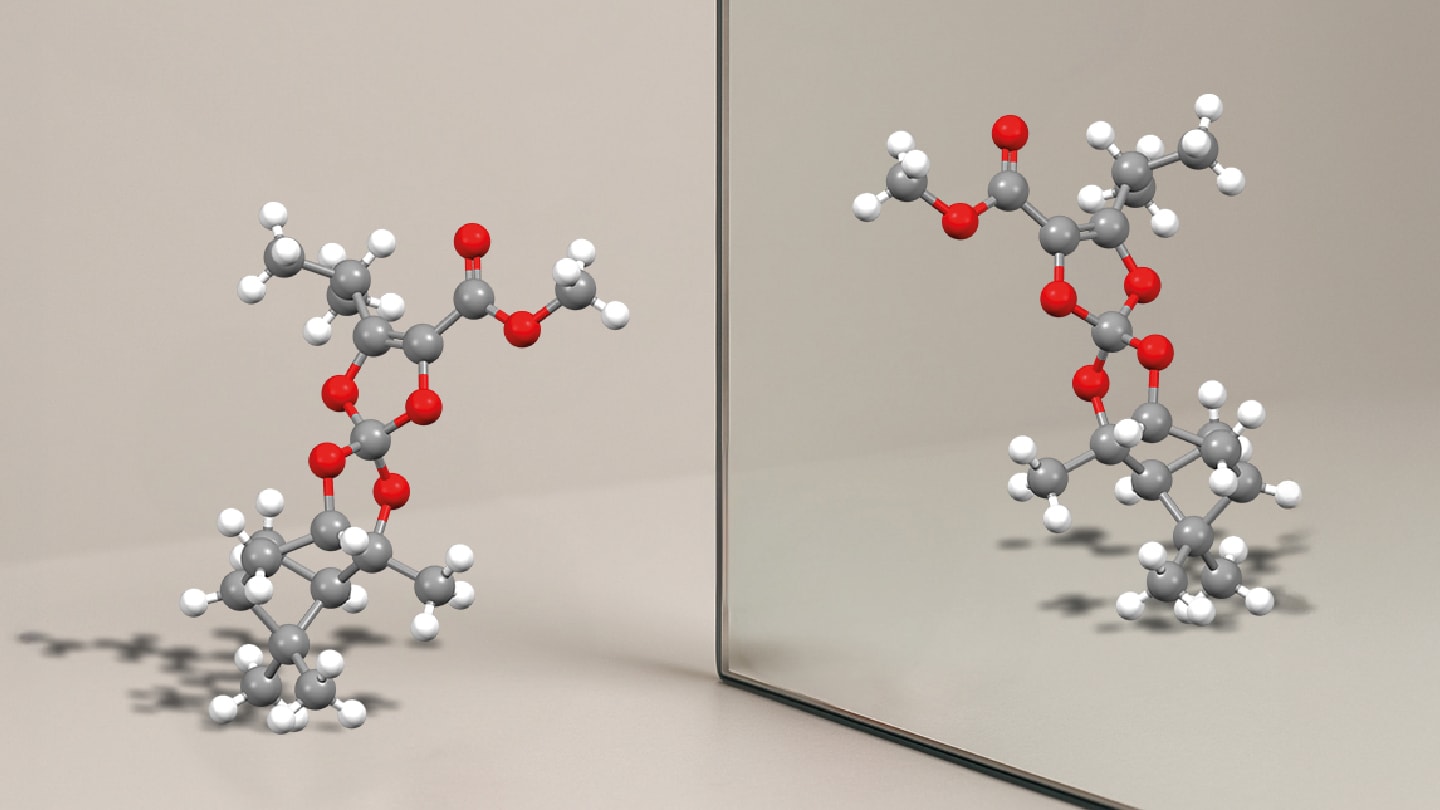
To evaluate the enantiomeric stability of the two synthesized compounds, the team – from the University of Geneva, Switzerland, and University of Pisa, Italy – applied dynamic high-performance liquid chromatography (DHPLC). By analyzing racemization kinetics under controlled conditions, they estimated that one scaffold has a racemization half-life of approximately 84,000 years at 25°C. The second, more flexible structure displayed a half-life of 227 days, still remarkably stable by pharmaceutical standards.
“Using dynamic chromatography techniques and quantum chemistry calculations, we have shown that, for the first molecule developed, it would take 84,000 years at room temperature for half a sample to transform into its mirror molecule,” said first author Olivier Viudes in a press release.
To confirm the absolute configuration and probe the optical activity of the enantiomers, the team turned to electronic circular dichroism (ECD) spectroscopy, supported by time-dependent density functional theory (TD-DFT) calculations. This combination of experimental and computational tools allowed them to unambiguously assign stereochemistry and model the electronic transitions responsible for the observed chiroptical properties.
The work challenges long-held assumptions about the instability of heteroatom-substituted stereocenters and paves the way for designing geometrically defined molecules with predictable behavior. “Molecules with this new type of stereogenic center had never before been isolated in a stable form,” said senior author Jérôme Lacour. “Their synthesis and characterization mark a major conceptual and experimental breakthrough.”
From a practical standpoint, the findings could inform the development of drugs where enantiomeric purity is essential. Long-lived chiral forms eliminate the risk of spontaneous inversion into inactive or harmful isomers.
“These novel stereogenic centers offer a new way of organizing molecular space,” added Gennaro Pescitelli, co-principal investigator from the University of Pisa. “They open up a whole new degree of freedom and imagination in chemical synthesis.”




