Alzheimer’s disease is characterized by the abnormal aggregation of amyloid beta (Aβ) into amyloid fibrils in the brain. Scientists have previously found that Aβ molecules bind to GM1 gangliosides on neural cell membranes – triggering the development of amyloid fibrils. However, the precise nature of GM1-Aβ has remained elusive. Now, researchers at the National Institutes of Natural Sciences and Nagoya City University, Japan, have conducted a study to explore the interaction of Aβ with GM1 glycolipids – and the work could help advance treatments for Alzheimer’s disease (1).
Using nuclear magnetic resonance (NMR) spectroscopy, the researchers studied the molecular structure, interactions, and dynamics of Aβ. However, when Aβ was attached to GM1, increased variability caused complications in analysis. To address this, the researchers used ultracentrifugation on specific membranes, followed by rapid freeze drying. “This innovative process enabled us to specifically capture and analyze the bound state of Aβ with GM1 membranes,” says lead author Maho Yagi-Utsumi.
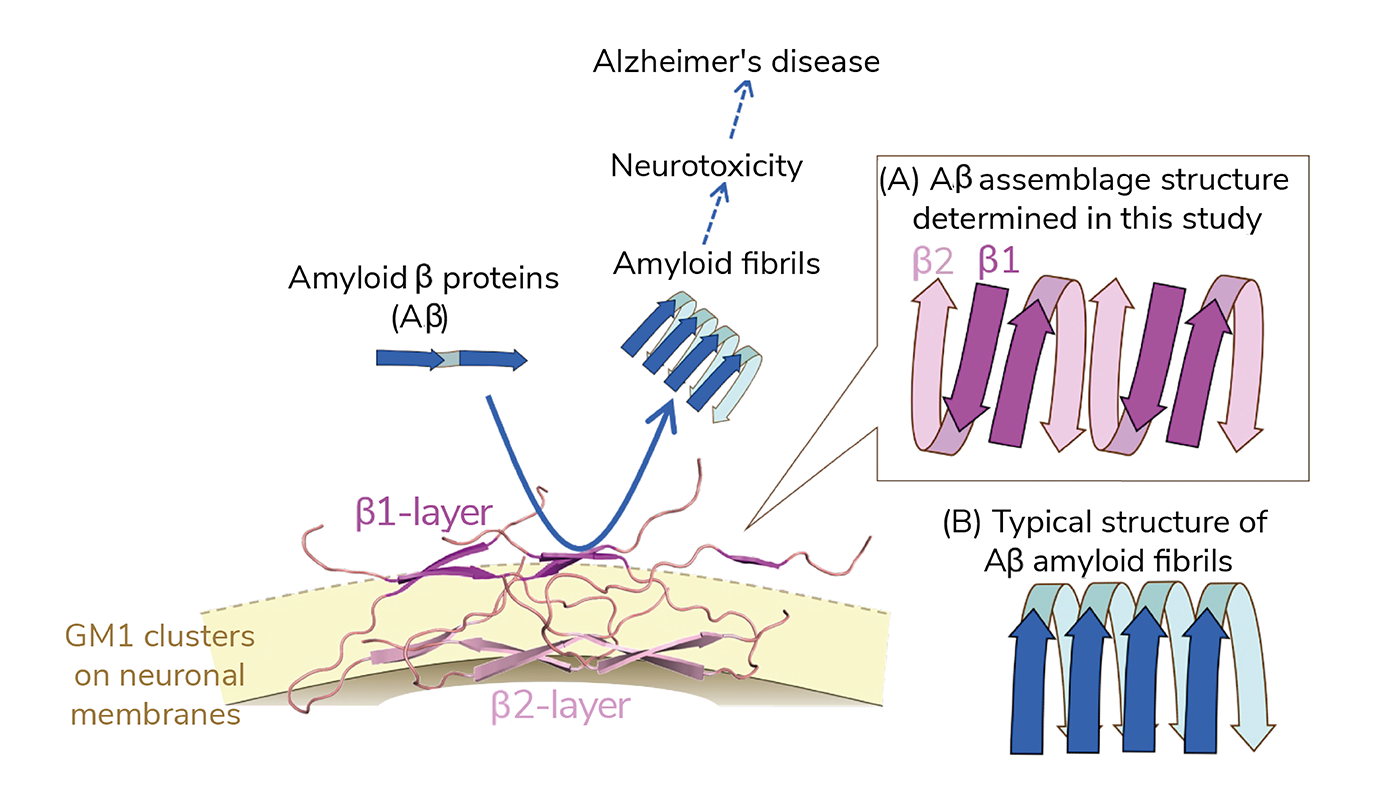
The analysis revealed that Aβ adopts a ‘U’ shaped structure formed of two Aβ layers – β1 (farther from the membrane) and β2 (closer to the membrane) arranged alternately. According to the team, the findings contradict previous research stating that Aβ amyloid fibrils align in a parallel direction. The exposed β1 layer on the membrane surface was found to act as a catalyst, significantly accelerating the fibrillation of surrounding Aβ molecules. Additionally, the team found that a unique anti-GM1-Aβ antibody specifically recognizes this region.
The team believes this study to be the first of its kind to identify the structural entity responsible for producing amyloid fibrils in brain tissue.
“This research offers insights into predicting the onset risk of Alzheimer’s disease and opening avenues for inhibiting its progression,” says Yagi-Utsumi. “Learning more about Aβ molecular structures introduces a novel strategy for the development of therapeutic antibodies targeting Aβ aggregates – providing exciting prospects for potential treatment of Alzheimer’s disease.”
Credit: Maho Yagi-Ustumi
References
- M Yagi-Utsumi et al., “The Double-Layered Structure of Amyloid-β Assemblage on GM1-Containing Membranes Catalytically Promotes Fibrillization” ACS Chem. Neurosci 14 (15), 2648-2657 (2023). DOI: 10.1021/acschemneuro.3c00192.




