
ARC wins MN Cup Cleantech Division (August 2016) (Credit all images: Andrew Jones)
I’ve always been fascinated with energy – in all forms. When I was about 10 years old, I bought my first solar cell from a surplus store and was able to use it to charge batteries, run motors, and power light bulbs – all from the sun. I was hooked, but I had so many questions: what was this energy and how could it be stored in a battery or in the chemicals in fuels and fireworks? Clearly, I needed to learn more about this magic, so I started reading about energy and experimenting with electricity and different forms of chemical energy. This was the early days of the internet, so I went to eBay.com and ordered potassium nitrate and sulfur (without my parent’s knowledge). I took charcoal from the fireplace and ground it up into a powder for a carbon source, then mixed it with the right ratio of sulfur and potassium nitrate to make an ancient recipe for black powder! When I lit it on fire it did what you might expect and burned incredibly fast. I tried lighting each of the components individually and they wouldn’t burn. I started to learn that the nitrate was providing the oxygen for the reaction and the sulfur was some sort of catalyst. The carbon was the source of energy. I was fascinated (and I still am today) by the amount of energy stored and released by the carbon in this reaction. This was the start of my lifelong fascination with energy and how to store/release energy with chemical reactions.
When I discovered chemical engineering in my freshman year at the University of Minnesota, Twin Cities, it seemed like the perfect blend of everything I was passionate about. Furthermore, I watched a freshman seminar by Lanny Schmidt who showed Paul Dauenhauer’s research (Paul was then a PhD student in Lanny’s lab). In a video, Paul was taking small pieces of wood and catalytically pyrolyzing them into hydrogen, fuels, and the building blocks of plastics. This was fascinating to me and seemed to touch on all the questions I had been grappling with as a young boy. How could you convert wood into all these products? Where did the energy come from? How do you control carbon bonds? I joined Lanny’s lab and had the opportunity to meet Paul. I learned more about catalytic pyrolysis and got a better understanding of how catalysts helped speed up and direct chemical reactions. With a yearning to learn more, I applied for and was accepted to the University of California, Berkeley’s graduate chemical engineering program under a National Science Foundation Graduate Research Fellowship. I spent five years learning catalysis from one of the best in the field: Enrique Iglesia.
Identifying a problem – and a need
After my PhD, I had the opportunity to start a company with a local businessman, Brad Cleveland. Brad and I both shared a passion for sustainability and entrepreneurship. The deal was that he would put all the money in and I would do all the work! Well, not exactly; Brad served as the CEO for the first two years. Together, we started Activated Research Company in July 2014, immediately after my graduation. We initially started working on fuel cells, but quickly realized the market wasn’t quite ready, so we pivoted to measurement equipment. In my PhD work, I had worked on the development of methanol to gasoline and methanol to olefins chemistry.
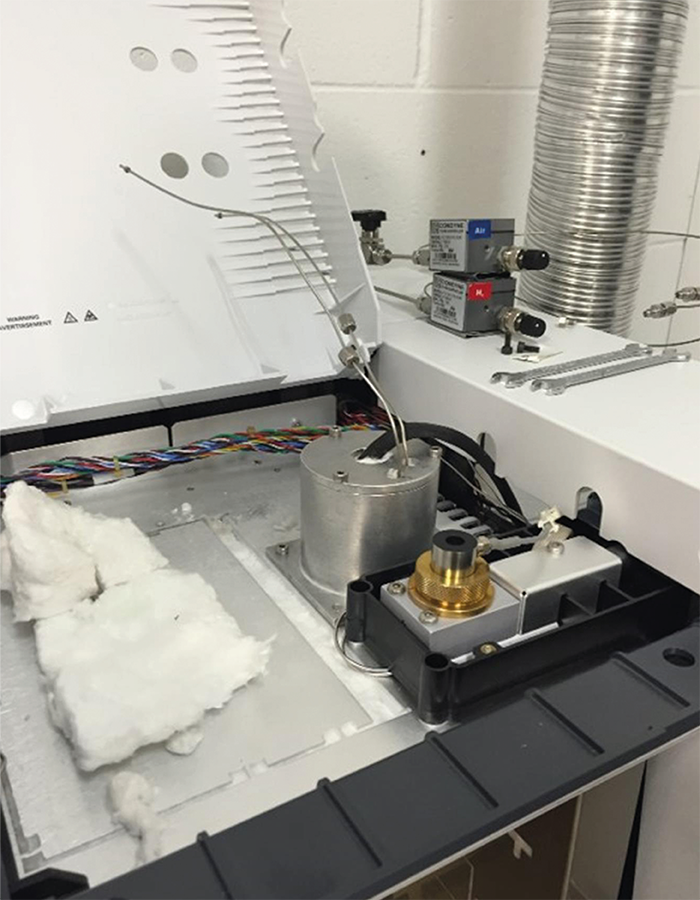
A real pain point in this work – and in most biofuels – is the analysis of a large number of oxygenates. The go-to method was to inject the fuels into a GC-MS and try to identify all the compounds. The concentrations were “guestimated” using response factors based on molecular weight or functionality. In some cases, where possible, chemical standards were used. But the reality was that chemical standards weren’t available (or stable) for many of the compounds; the identifications were tenuous and there were so many types. It was effectively impossible to get complete quantification and carbon closure, making accurate research and relevant conclusions difficult to come by. At the same time, I visited Paul Dauenhauer, who had just moved back to MN as a Professor in the very same department where we met. He also shared the same frustrations with GC-MS and had an idea for converting compounds to methane so we could just count carbons rather than grappling with unknown response factors. Together we thought it would be great to create a small catalytic device that could convert all organic molecules after GC separation into methane before detection. Knowing very little about GC and even less about the different markets for GC (not a great way to start a company!), I went to work building the world’s first metal 3D-printed reactor for GC. (To be honest, the first design wasn’t 3D printed. It was a large, bulky mass of tubes and fittings that sort of worked, but gave horrible peak shapes and had severe issues with pressure drop and dead volumes.) Three-dimensional metal printing was very new at the time, but seemed to offer limitless possibilities that would allow me to construct a reactor the “right” way. From there, the Polyarc was born.
The Polyarc is a sequential combustion–reduction reactor that converts (almost) any organic molecule to methane(s) and their associated byproducts. The purpose is to give equimolar carbon response to GC. Molecules are injected into a GC, separated by the column, and then transferred into the Polyarc where they first undergo oxidation/combustion with a small amount of air (2.5 sccm), then reduction with hydrogen (35 sccm), before being transferred to the detector; for example, the flame ionization detector (FID). The carbon atoms in the molecules are converted to CO2 in the oxidation step, and the CO2 is subsequently reduced to CH4. Since the FID only sees reduced carbon and is insensitive to water, nitrogen, and sulfur compounds, the resulting signal in the FID is proportional to the number of carbons/CH4.
The tool was designed to allow scientists to analyze very complicated mixtures and get accurate quantification. We started by targeting users in catalysis and biofuels, and that continues to be a big market. However, we also realized the tools were useful in other areas, including petrochemical, refining, flavors and fragrances, transformer oil gas analysis, paints and coatings, surfactants, polymers, and more. Furthermore, the tool also made previously low- or negligible-response molecules increase in sensitivity thanks to the transformation to methane. Molecules such as CO, CO2, formic acid, formaldehyde, carbon disulfide, carbon tetrachloride, and more started to have the same sensitivity as methane.
I believe this is just the start of a new paradigm in GC, something we call “reactive chromatography.” We can use selective chemical reactions to transform molecules before, after, and during chromatography to influence selectivity and sensitivity.
We realized that a lot of folks were just interested in replacing their methanizers with the Polyarc; shortly after, we invented the Jetanizer, which is just the reduction portion of the Polyarc shrunken into the size of an FID jet. This allows folks to analyze CO, CO2 (and even formaldehyde) just by replacing the jet in their FID. It has become a very popular product.
Early prototype (Sept 2015)
Development Breakthroughs
There were several breakthroughs in the development of the Polyarc. The development of a combustion catalyst that was fast, efficient, and resistant to poisons was key. The reduction catalyst has also been incredibly successful and robust. The use of 3D printing was risky at first, because it wasn’t well known if there would be porosity or what minimum feature size could reliably be produced. There were also novel welding techniques to approach zero dead volume and make the device easy to integrate.
A major breakthrough came when we developed a novel welding technique to bind small metal capillaries to the 3D printed body. The most significant breakthroughs, however, were in the market. Once we realized that the Polyarc was at least ten times more robust than current Nickel-based methanizers and that we could use the Polyarc to replace them in difficult situations. Or when the National Renewable Energy Lab used the Polyarc to get 90%+ carbon closure on a pyrolysis fuel, when the previous best was much lower and included exhaustive calibration (this was calibration free).

GCC best new product showcase (2015)
“The learning was fast and brutal”
I already admitted that I knew very little about the analysis and chromatography market when I embarked on our endeavor. As a chemical engineer and chemist that used GC as a tool, I was familiar with its strengths and weaknesses and necessity in chemical research. I wanted to improve the experience for other scientists and engineers like me, who wanted to use it as a tool and get data as quickly and efficiently as possible. I soon realized how massive and widespread the GC market is. It was incredibly exciting learning from chemists in industries that spanned pharmaceuticals, oils, foods, fragrances, fuels, research, biology, fertilizers, and more.
The development of the Polyarc took about one year from start to finish. Then another eight years to market, sell, and educate. I followed lean entrepreneurship principles to get to a prototype quickly and test the MVP with real customers. The learning was fast and brutal. My first prototype broke at a customer site because of a weak weld. The second gave poor peak shapes. The third didn’t seem to combust the molecules, and by the fourth I realized that chemical standards might be inaccurate as well. I started making my own and realized many of the standards we rely on can have inaccurate concentrations or issues with inlet/column discrimination. We started to learn which industries this would work for and which it wouldn’t. I learned that much of GC is highly regulated, and that even if there was a better way, it probably wouldn’t be used until the standards/regulations were rewritten. This process could take years or decades – just look at the number of methods that still rely on packed columns!
Of course, as a startup you need to get to revenue as quickly as possible if you want to survive. Also, many of the large companies don’t want to purchase from a startup that’s only been around for a few years, because many don’t survive. In short, you really need a few years runway, just to get to initial sales. I have Brad Cleveland and his wife to thank for providing that runway. I was also fortunate that our first employees were great – all incredibly passionate about our mission to transform analytical chemistry (Kim Herzog, Raj Mehta, Amy Jacobson, and Tommy Saunders to name a few).
The amount of regulation in the industry was a large, unexpected issue. The discrimination within GCs was another. We expected a flurry of early adopters and then a gap between them and the early majority – the so-called chasm of death. And that is exactly what we saw; however, it took longer than expected to get into the mass market and, in many industries, we are still stuck with the early adopters. I hope and expect that will change with the backing of Shimadzu Corporation.
Back in 2020, Shimadzu announced that it would be offering Jetanizer to its customers in the US and subsidiaries around the world. This year, Shimadzu acquired both our Jetanizer and Polyarc technologies, which are now available globally. It is difficult for a small company like the Activated Research Company (ARC) to sell and support products – as fantastic as I believe them to be – worldwide. So I was very excited by the prospect of selling these products to Shimadzu and collaborating with them further. Shimadzu brings a worldwide network of sales and support – as well as a reputation for excellence that can give the Polyarc and Jetanizer the opportunity to flourish. Though there was an initial feeling of sadness, it was quickly overwhelmed by excitement over the potential for growth of these products and the potential for future product development. ARC is still operating, thriving, and producing new products that we hope will continue to change chromatography – perhaps even contributing to the development of the next generation of energy.
Let’s breathe life into GC
I have read many articles about the “maturity” of GC – some even claiming that GC is a dying technique that will be replaced by mass spectrometry entirely. Clearly, GC is commonplace in research labs, and there has been a lack of significant innovations over the past 30 years (except the Polyarc/Jetanizer!). The 5890 in my lab from the 1980’s is remarkably similar to my 7890 in form, function, and performance. However, I think it is naïve to assert that innovations aren’t necessary or won’t happen. Nature’s chemical complexity necessitates separation technologies, in my view. And I don’t see a future where a mass spectrometer can accurately identify and quantify the thousands of molecules in a strawberry or biofuel without chromatography. (I realize some may disagree since the separation can happen in multiple quadrupoles; however, it’s important to note that the complexity, cost, and size of these technologies are significant barriers).
I hope that the GCs we use today look like relics in 20 years. The air bath oven is an energy hog – and it is slow and bulky. The analog circuitry in the FID has remained largely unchanged in the last 60 years since its invention. The column is still a fiber of glass coated with polyimide. There is so much room for improvement. And future scientists will benefit immensely from this work. Let’s just hope it is groundbreaking enough to get the regulations to change…
My experience tells me that the maturity of the GC market can create large barriers to new products. Nevertheless, it is clear that there is a healthy ecosystem of early adopters and champions who help drive new innovation – even in GC.
