Every year, as part of our undergraduate course – Analytical Methods in Organic Chemistry – one or two hundred students from molecular life sciences and biotechnology are introduced to the wonders of mass spectrometry (MS). In the education space, it can be tricky to keep up with the fast pace of MS advances, and we’re pleased when we are able to widen the experience to prepare students for the real world. But before we share the details of our latest educational endeavors, a little history... Back in the 1990s, electron ionization (EI) was the most accessible technology in educational circles, and so the focus of the MS course was on both ion formation and interpretation of EI spectra. Over the years, gas chromatography (GC)-MS systems with EI became more affordable and entered into the practical course program; nowadays, we have two GC-(EI)MS systems permanently situated in our education building.
Introducing ESI
Over the same period of time, the course had to be updated with the theory of soft ionization techniques, such as electrospray ionization (ESI; (1), (2)) and matrix-assisted laser desorption ionization (MALDI). Unfortunately, hands-on practical experience was not feasible, so students were exposed to ESI through visits and demos at a research lab. Given the importance of ESI-MS in molecular life sciences and biotechnology in general, this was definitely not an ideal situation. Thanks to recent developments in affordable and transportable quadrupole-based ESI-MS systems (for example, the Acquity QDa detector from Waters, and the Microsaic 4000 MiD from Microsaic Systems), it is now feasible to purchase a system exclusively for educational purposes. We’ve done just that and it enabled us to develop a dedicated undergraduate practical course on ESI-MS. Transportable ESI-MS systems are typically marketed as selective detectors that can complement or replace photo diode array UV-Vis detectors in liquid chromatography (LC) systems. But for educational purposes, we have been using a quadrupole ESI-MS system as a standalone unit in a flow injection analysis set-up, which consists of an old isocratic LC pump and a manual injection valve with an inline filter between the injector and the MS (see Figure 1 and “Setup”). Our new system means that students are exposed to both GC-(EI)-MS and ESI-MS in practical hands-on courses. And our MS course objectives have been updated to ensure that students: i) learn and understand the differences between EI and ESI ionization in terms of the type of ions formed, the degree of fragmentation and the structural information obtained; and ii) understand the opportunities and limitations in the applicability of EI- and ESI-MS for the characterization of (bio)organic (macro)molecules and mixtures thereof.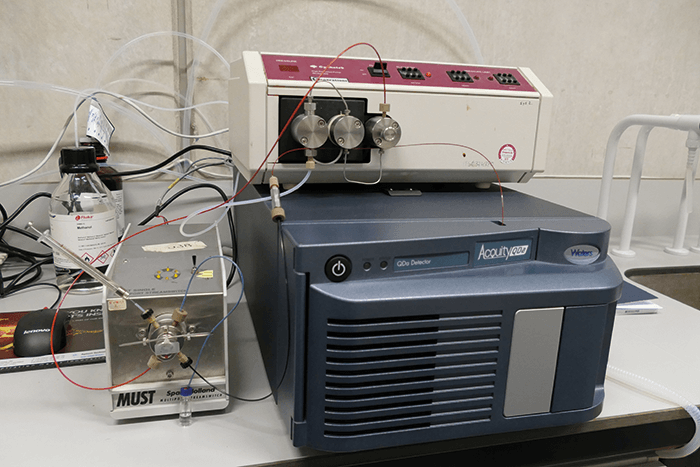
We use a model Acquity QDa (Waters) single quadrupole MS with a mass range of m/z 50-1250, operating in alternating positive (capillary voltage 1.2 kV) and negative (−0.8 kV) ion mode at a source temperature of 120 °C, a probe temperature of 600 °C and a cone voltage of 15 V. The LC pump is an old Gynkotek model 300 operated at 0.4 ml/min. The injection valve has a volume of 5 µl. Following each injection, raw data are combined and background subtracted in Masslynx 4.1 (Waters) to obtain high-quality mass spectra for student interpretation. All chemicals are from Sigma-Aldrich, except for the aliphatic carboxylic acids and the amino acids, which are from Alfa Aesar. Standards are dissolved in HPLC-grade methanol (Rathburn) at 100-200 µg/ml, except for phenylalanine, lysine and PEG 400 (20, 20 and 50 µg /ml, respectively).
Covering all bases
Thanks to the flow injection set-up and the very short start-up time and robustness of the quadrupole MS, we can typically get groups of 2-4 students to perform each of the following three experiments in under one hour of instrument time. Mass spectral interpretation and reporting requires extra time, of course.Low molecular weight compounds
We prepared a number of mixtures based on their potential to highlight the spectral differences that will be typically obtained when analyzed by both EI and ESI-MS: primary amines (octylamine, decylamine, dodecylamine), secondary amines (dipropylamine, dibutylamine, dihexylamine), primary alcohols (decanol, dodecanol) and primary carboxylic acids (octanoic acid, decanoic acid, dodecanoic acid). As expected, the primary and secondary amines ionized very well in positive ESI, yielding intact [M+H]+ ions (Figure 2a/b). On the other hand, in EI the mass spectra of the primary amines are dominated by the homolytic fragment ion at m/z 30, thereby losing the entire molecular weight information.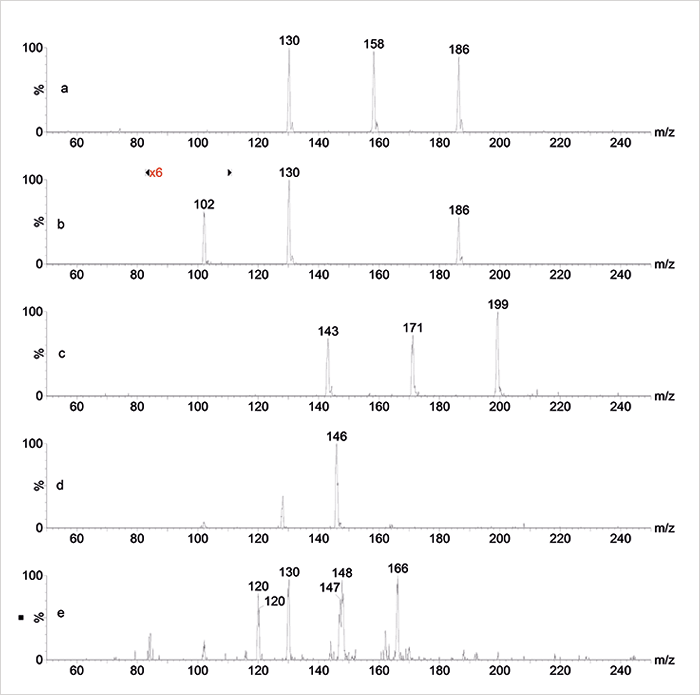
The primary alcohols are difficult to ionize in positive or negative ESI and no information is obtained at all; in EI, extensive fragmentation and water loss occurs and the molecular ion cannot be seen either. The carboxylic acids hardly ionize in ESI unless the negative ion mode is selected and the pH is adjusted above the pKa with some ammonia: the intact [M−H]– ions obtained allow a straightforward assignment of the mixture (see Figure 2c). When analyzing the mixture of amino acids (lysine, glutamic acid, phenylalanine) GC-MS obviously fails, while ESI in both positive and negative mode does a complementary job: negative ion ESI highlights the [M−H]– ion of glutamic acid at m/z 146 (see Figure 2d) while [M+H]+ ions of phenylalanine, glutamic acid and lysine can all be found in the positive spectrum at m/z 166, 148 and 147, respectively – including a loss of water from protonated glutamic acid at m/z 130 (Figure 2e).
In all these cases, we can pose a number of questions to students, for example:
- How many compounds are present in the unknown mixture?
- Which ions are the (quasi) molecular ions?
- Are there any sodium or potassium or solvent adduct ions?
- Is there an odd or even (including zero) number of nitrogen atoms?
- What would be a possible elemental composition?
- Are there fragment ions and if yes, which cleavage reaction occurs?
Synthetic polymers
Poly(ethylene glycol) is easy to ionize in positive ESI and typically yields a series of intact [M+Na]+ ions separated by the monomer mass of 44 Da (Figure 3). High molecular weight PEGs may extend beyond the upper mass range of the quadrupole and, in addition, yield multiply-charged ion series causing too much confusion for the students, so typically PEG400 or 500 would be appropriate. Now the same questions as above will challenge the students a bit more since they are not really familiar with polydisperse polymer mixtures. Following some hints, they should be able to identify the polymer and discover that we are facing a mixture of single-charged polymer ions having the general formula [A+(C2H4O)n+B+C]+ in which A and B represent the end-groups of the polymer chain and C a species that provides the positive charge (proton, sodium, potassium). Finally, they may come to the conclusion that the spectrum represents polyethylene glycol having an average degree of polymerization of 10 and a value for A+B+C of 41 Da, which suggests a PEG400 having H and OH as end-groups and sodium as the cation.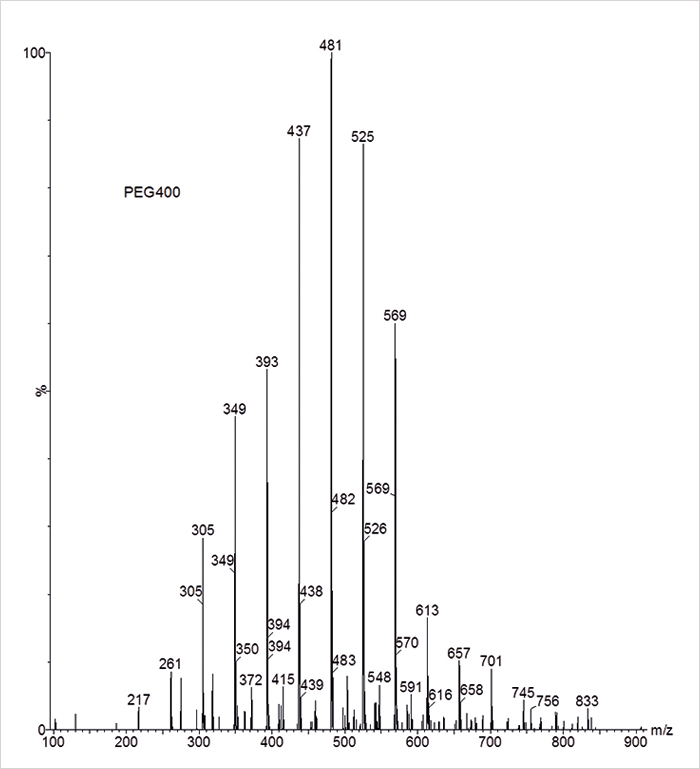
“We found that the relatively high accuracy of the calculated protein mass is very often a pleasant eye-opener for students”
Carbohydrates and proteins
Low molecular weight oligosaccharides such as maltose (MW 342), raffinose (MW 504) and maltotetraose (MW 666) show simple single-charged [M+Na]+ ions in positive, and [M−H]− ions in negative ESI (see Figure 4a/b). Proteins, on the other hand, are rather confusing – especially for those students who have yet to study the theory of protein ESI-MS! Clearly, the MS system is also pushed to (and beyond) its limits because of the fact that proteins show multiply-charged [M+nH]n+ ion series in the range of m/z 700-1500 – that’s beyond the upper mass range and the nominal mass resolution of this small quadrupole MS. The mass spectrum of ubiquitin (see Figure 4c) is rather noisy, as expected, but shows a sufficient number of multiple-charged ions for precise calculation of the protein mass based on the assumption that adjacent multiple charged ions differ by one charge state only; using the three most intense multiple charged ions, the deconvoluted protein mass would be 8566 Da, which is only 1.5 Da higher than the real molecular weight of ubiquitin.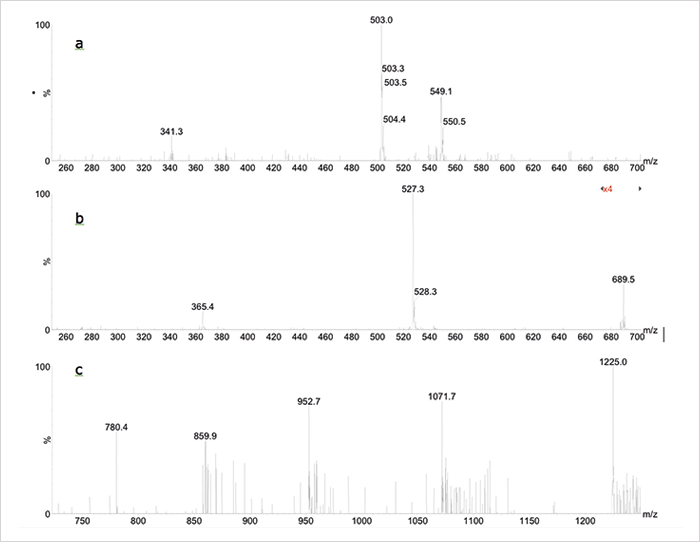
Challenging questions for students:
- How many substances are present in this sample?
- Which ion(s) is/are the (quasi) molecular ion(s)?
- What is the molecular weight of the substance(s)?
Give it a try?
If you were wondering, the flow injection ESI-MS set-up is simply switched off when not in use and powers up in approximately five minutes – including auto-tuning and mass calibration – so the system is really education-ready. And of course, the same set-up can be applied as a detector to complement an LC-UV set-up in chromatography courses. We hope we’ve shown here that it’s possible to develop a highly affordable and straightforward solution for undergraduate practical courses in mass spectrometry. Why not drag your MS courses into the 21st century? We’d love to hear how you get on. Michel WF Nielen, Pepijn Geutjes, Barend van Lagen, and Teris A van Beek are all based in the Laboratory of Organic Chemistry at Wageningen University, The Netherlands.References
- JB Fenn et al, “Electrospray ionization for mass spectrometry of large biomolecules”, Science, 246, 64–71 (1989). JB Fenn et al, “Electrospray ionization-principles and practice”, J Am Soc Mass Spectrom, 9, 37–70 (1990).




