Mass spectrometry is riding high at the moment; increasingly, it is the method of choice for identification, characterization, and quantification of all kinds. But I know of a simpler, faster, cheaper and greener method; an approach that can “do the mass spec” without using a mass spectrometer; a mass-spec killer! It is called laser ablation molecular isotopic spectroscopy (LAMIS) and is the culmination of a career spent in the pursuit of new analytical methods using lasers. More details later.
LAMIS is the latest chapter in a career in laser research that dates back to the 1970s. It really began when two of the pioneers of atomic spectroscopy – James Winefordner and Gary Hieftje – impressed upon me in my undergraduate and PhD years that direct solid sample analysis was the ‘Holy Grail’. I became aware that others had proposed (as early as 1962) that a viable method of achieving that goal was laser ablation, which is the process of removing minute particles of mass from a sample via a highly focused laser beam. By combining these two pieces of information, I secured funding from the US Department of Energy (DoE) to study laser ablation for chemical analysis. This was at the Lawrence Berkeley National Laboratory (LBNL) in 1982. My proposal was that a green technology (which at the time was an emerging issue) could be developed for rapid, direct, solid-sample chemical analysis, eliminating laborious sample dissolution procedures. But, looking back, that was just the tag line – my real desire was to unravel the fascinating physics behind the micro-explosion that occurs when you blow things up with a high-powered laser. In those first years, I was just completely enamored by the process and having a real ball.
Back in the early 80s, commercial instruments were rudimentary and more like laser beam delivery devices than analytical measurement instruments. Instead, you sourced a laser, found a detector and set it all up on an optical table, aligned everything by hand (there was no software, as such) and recorded most of the data on an oscilloscope. And we say “those were the days”? Nevertheless, that early work laid the foundation for understanding the parameters that affect the ablation process. Over the subsequent 30-some years, my group at LBNL has immersed itself in the fundamental mechanisms of the explosive ablation processes. We have described mass removal rates, fractionation (in the form of preferential vaporization), matrix effects and plasma spectrochemistry. Perhaps the most important advance was when we demonstrated the influence of laser-pulse duration on analytical performance; this in particular, and our work overall, convinced people that laser ablation could not be treated as a “black box”. It is a delicate process in which each of the fundamental parameters can have a tremendous impact on chemical analysis; as with any other analytical instrument, a stable system and a reliable method are essential.
Data for skeptics
The whole laser ablation chemical analysis field has been criticized from the beginning. You know, some people just don’t like change or progress – it took almost 20 years for typewriters to be replaced by computers, because the masses just couldn’t accept that computers were going to be so useful. So, when laser ablation technologies (see, “Laser Ablation Chemical Analysis”) threatened to replace traditional chemical dissolution techniques - there was a lot of resistance. Two particular criticisms kept being repeated. One was, “You don’t get representative sampling.” We addressed this objection with very high repetition rates using femtosecond pulse lasers. This allowed us to sample more mass, take a signal average, and integrate any inhomogeneity. Not only could we describe the bulk sample composition, we also provided information on those inhomogeneities – knowledge that can be very valuable to industry. The second criticism was, “You don’t have standards.” Our response was that in most industries the same product is made continuously and the desired tolerances are set; thus, the process contains its own standards.Laser Ablation for Chemical Analysis
Laser ablation is the removal of a small quantity of mass from a sample surface using a focused, pulsed laser beam. After the laser pulse, a high-temperature (>15,000 K) plasma rapidly rises from sample surface and into the ambient medium followed by fine particle ejection.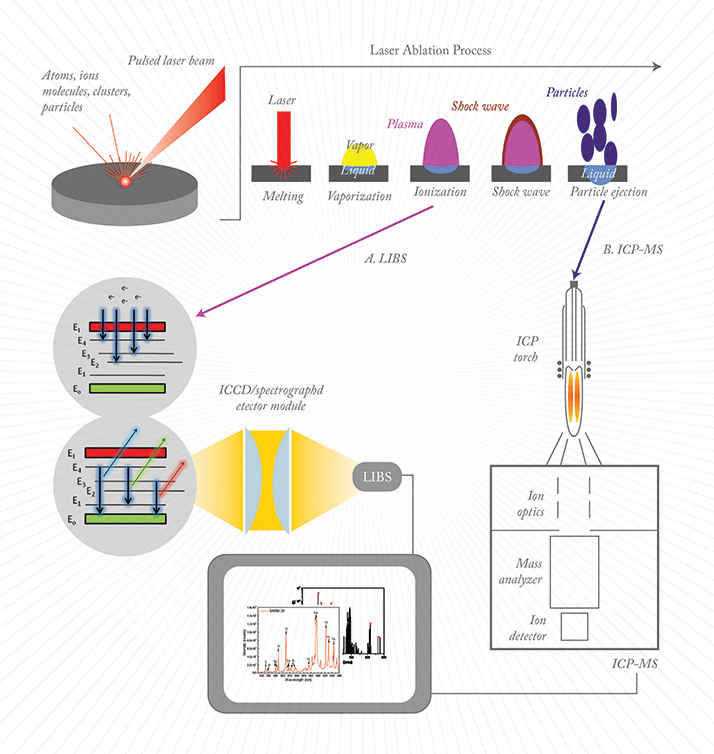
A. Laser Induced Breakdown Spectroscopy (LIBS) is a rapid chemical analysis technology that analyses the light emitted from the micro-plasma explosion. Continuum light is emitted during the early stages of plasma cooling process (< 200–300 nsec) followed by discrete atomic lines at around 1 µsec. Light emissions are collected by the ICCD/spectrograph detector module, followed by spectral analysis by system software. LIBS spectra are displayed and subjected to qualitative and quantitative elemental analysis.
B. Laser ablation (LA)-inductively coupled plasma (ICP)-MS analyzes the ablated particles by transporting them to the secondary excitation source of the ICP-MS instrument for digestion and ionization. The excited ions in the plasma torch are subsequently introduced to a MS detector for both elemental and isotopic analysis.
Besides overcoming these practical challenges, we have used more powerful data analysis and chemometrics to address other challenges. For example, we – like everyone else – had to rely on univariate analysis, using the area under the curve to calculate the concentration. Now, we have complex spectra with thousands of lines and software can determine whether those lines really are iron or, say, iron being interfered with by titanium. Of course, this is a general phenomenon. The power of modern computers and chemometric algorithms mean we can all do so much more with our data, allowing us to address what were previously considered to be problems. Take nuclear magnetic resonance or near-infrared spectroscopy: neither would be viable without sophisticated algorithms behind them.
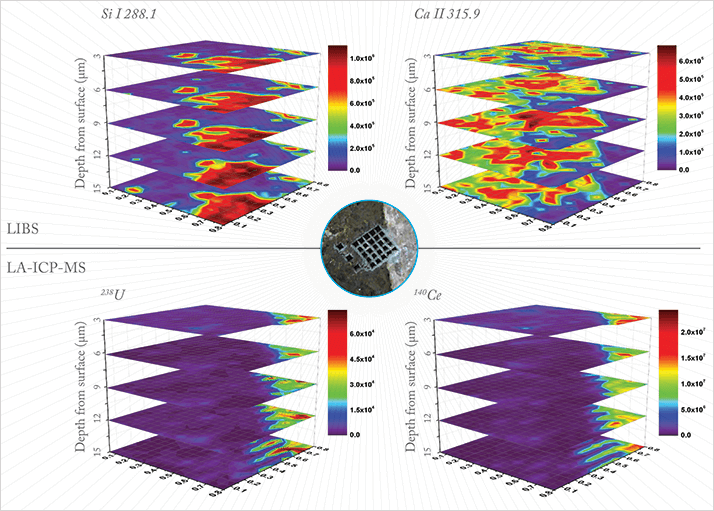
People have criticized the technology my entire career and you can talk until you’re blue in the face, but they won’t believe you. The only way to win the arguments is with data. So we made measurements and we shared results and, as the number of published papers grew, it became harder and harder to deny that the technology has distinct merits. Amongst other successes, my group has achieved world records. for spatial resolution (450 nm) and for detection of 220 attograms for elements in geological samples using a single femtosecond laser pulse with LIBS. That’s no mean feat. No other technology can provide such detection capability at atmospheric pressure, without sample preparation, and in real time. I rest my case!
In the process of academically proving the veracity of laser ablation, I began to understand its real significance and potential from an application point of view. Our understanding of the parameter space coupled with the improvements to laser and detector hardware created the perfect storm. And so, in 2004, a few of my PhD students helped me found Applied Spectra (see sidebar “Commercially Applying Spectra”, page 29) to commercialize the technology. We had expertise in laser ablation chemical analysis using LIBS, in addition to laser ablation with ICP-OES and ICP-MS and successfully transitioned this knowledge into the manufacture of chemical analysis instruments.
I’m doing exactly what I set out to do when I submitted that funding proposal to the DoE; selling instruments around the world that address some significant analytical problems. That’s despite the fact that I started with an obsession for blowing things up with a laser beam and had no real clue initially about how to fulfill the aims set out in the grant application.
In truth, we still don’t fully understand the physics. But we can accurately replicate the process and that’s key. Once you define a method and achieve reproducibility, you have a viable analytical technique, which can finally be treated as a black box by the customer. We help our customers to set up reproducible methods for their application and, for the most part, they are not interested in what laser and what detector is used - they just want to be sure that it will quickly measure 10 ppm mercury in soil or warn them of sodium contamination. Industrial analysis has clear demand: simplicity and speed.
In a wonderful role reversal, I’m learning more and more about potential markets from my old PhD student Jong Yoo, who is now Vice President of Technology and Marketing at Applied Spectra. LIBS is gaining real traction in a number of industrial sectors, such as solar, semiconductor, environmental and materials, and the list is growing. That’s not too surprising since the technology is able to measure pretty much any element on the periodic table. Put simply, if you shine a laser beam at something, you get an answer. NASA have used the same technology on the Mars Curiosity Rover (see page 16) and, while I wasn’t directly involved in that project, many of my colleagues were. I visited Los Alamos to see the prototype and discuss it with the team and now, my group is working with NASA to look at the next generation of this capability. Which brings me to LAMIS.
Mass spec killer
Most of my career has been pretty fortuitous: my approach is to delve blindly into something that I know very little about, learn from others, and see what happens. That’s exactly how, two years ago now, we made a breakthrough in the ability to measure isotope ratios in our ablation plasmas. Everything just seemed to fall into place. When you create a laser spark, you get a lot of background – white light plasma – which changes as a function of time after the initial spark. We usually try to set our detector gate or timing to get good signal-to-noise for the elements, whether we want either atomic or ionic lines, but there are always background molecular spectra. As hot ions and atoms collide with the oxygen and nitrogen in air or different species within the sample, they form molecular species, as the plasma cools. We were brainstorming about this and someone reminded the group that there is good isotopic information in molecular spectra (a fact that is well known); instead of trying to get rid of this “noise”, we said, why not try and enhance it? Sure enough, when we got down to understanding the fundamentals of molecular spectra, tweaked the system and used samples with different isotope ratios, we saw beautiful isotope splitting.Telling people that “LAMIS is mass spectroscopy without a mass spectrometer” raises a lot of eyebrows. Do I believe it? I think that I do - you just never know what’s possible (for instance, who would have ever thought that we would have a laser on Mars?) In reality, we have a long way to go with our technology. Mass spec has been around for a hundred years, ICP-MS since the 1970s, but LAMIS is only two years old. We’re certainly going in the right direction. We’ve published seven papers in those two years (the first three papers published in 2011 won best papers in Spectrochimica Acta) and LAMIS won a 2012 R&D100 Award and technology recognition from NASA. Right now, we’re trying to tailor the laser plasma for particular molecular species in order to improve the precision and sensitivity of our analysis. We’ve seen the potential of measuring isotope ratios, and simply must gain a better understanding of the chemistry. We are investigating how these molecules form in the laser plasma and how we can prevent interference. These are big hurdles, but the very same problems had to be overcome in mass spec: that’s why collision cells were developed.
To infinity and beyond…
Like all new technologies, LAMIS will have to prove itself; the scientific community and time bear the challenge. The advantages are clear – measure isotope ratios in real time, without sample preparation, and without a mass spectrometer (measuring isotopes at atmospheric pressure). Initial interest has come from within nuclear, geological and medical applications. With improvements in precision and sensitivity allowing measurements in these applications areas, it will not be long before commercial LAMIS instruments will be available to these (and other) industries, as modifications to our existing LIBS systems. In addition to analytical technologies, I’ve participated in several esoteric projects. One was the discovery of the world’s smallest ‘nanowire’ laser. Another was development of an on-line laser ultrasonic sensor to help the paper industry. I even got involved in fabrication of high temperature superconducting (HTSC) thin films using pulsed laser deposition (PLD). In two years, my team achieved a world record critical current in one of our superconductor films. All further evidence of my love affair with lasers...Looking to the future of LIBS, I can see laser ablation being taken into a whole new realm by harnessing near-field scanning optical microscopy. Pretty much everything that I’ve done has been in far-field optics, where focusing the laser down to spot sizes of half the wavelength is pretty difficult. With near-field optics, the technology (whether it’s LIBS or laser ablation with some secondary source) can be used on the nanoscale. That’s a whole new world of physics that someone is going to need a lot of time to study and understand – and it probably won’t be me. Near-field optics is a different way of using light; the laser is focused through an optical fiber that must be nanometers away from the surface. While we have been working on it for about five years and can perform ablation at the nanoscale, we have not yet been able to detect a signal. We don’t even know if the physics are the same. This is the next generation – literally; it’s for the young people who have a lot more time and patience to dedicate their careers to understanding this complex and exciting science.
As for me, I will continue to approach technologies, concepts, and devices in my own way. That usually means without a huge amount of expertise but with the attitude that I can do whatever anyone else can do but in my own different (not necessarily better) way. I owe my accomplishments to the many people that have entered my life and tolerated my craziness, from my initial mentors to the many students and colleagues who continue to make sense of my passions for learning and lasers. My devotion to lasers is ongoing: when you see the ways that they have changed society, from surgery to manufacturing to cutting, welding, and scribing, I see no reason why they will not play a dominant role in analytical chemistry. People are beginning to see the light. Rick Russo is leader of the Laser Spectroscopy and Applied Materials Group at LBNL (teamd.lbl.gov), and CEO and founder of Applied Spectra Inc. (www.appliedspectra.com).




