The problem
There is a demand for analytical tools that can allow scientists, medical doctors, and regulatory agencies to rapidly diagnose, prognosticate, or monitor a system under investigation. However, most of the “traditional” workflows for analyzing pesticides in food and environmental matrices, xenobiotics in biofluids, and metabolites in tissue samples (among others) are slow, burdensome, and difficult to operate. Therefore, analytical tools intended for contemporary problems must not only be rapid, but simple, cost-effective, deployable, capable of providing data in real-time (or close to it) and, preferably, allow for high-throughput analysis. Furthermore, these technologies must be sensitive and selective, and be easily coupled to state-of-the-art detection systems, such as mass spectrometers – all while guaranteeing minimal contamination and long-term operation. Finally, these tools must produce reliable results and, if possible, allow for multiple substances to be monitored simultaneously.Background
For nearly 30 years, Janusz Pawliszyn and his research group at the University of Waterloo have been developing analytical technologies that can facilitate the rapid and “green” determination of target compounds in complex matrices, particularly in the field of sample preparation. Although his group’s most widely-known contribution came in 1989 with a solid-phase microextraction (SPME) fiber for gas chromatography (1), they have also developed a number of tools over the past two decades that, although less widely acclaimed, deserve equal or greater attention.To ensure an adequate understanding of recent SPME developments, a quick review of this fascinating technology is in order. Simply put, an SPME device consists of a solid support with a portion coated with a solid or liquid polymer phase (see Figure 1). Contrary to popular belief, the solid substrate can be made of any material (including plastic, metal, metal alloys, paper, or wood) and can embrace diverse geometrical configurations (for example, wires, meshes, pins, rectangular blades, or membranes) to facilitate target applications (2). Though SPME was originally intended for equilibrium-based extractions of volatile and semi-volatile compounds present in the headspace of liquid/solid samples enclosed in a vial/container, continuous technological improvements have expanded its applicability to the direct extraction of target analytes from complex liquid and solid matrices, such as biofluids and tissue. As a matter of fact, improved (thinner and more homogenous) matrix-compatible coatings coupled with state-of-the-art mass spectrometry have made the use of pre-equilibrium SPME increasingly relevant, which has in turn enabled shorter extractions with adequate limits of detection. Certainly, matrix-compatible coatings have played the most important role in allowing SPME to be used for otherwise extremely challenging applications, as they help prevent matrix components from accumulating on the coating surface; matrix component build up can result in significant matrix effects or poor reproducibility, despite providing adequate analyte recoveries (3). In addition, the use of SPME devices with large surface areas (for example, the rectangular blade, see Figure 1) results in increased extraction rates, which allows lower limits of quantitation while maintaining rapid analysis. Furthermore, such SPME devices can be equipped with a holder that can accommodate 96 blades, which further reduces the analysis time required for each sample (4). Notwithstanding the exceptional advances in SPME technology, their interface with sensitivity and selective detectors, such as mass spectrometers, has been traditionally performed via chromatographic separations. Consequently, analytical results are generated at a speed that is strictly dependent on the length of the chromatographic run, which limited the technology’s applicability to rapid and on-site screening. It is therefore not a surprise that one of the most lauded features of SPME technology is its ability to be directly interfaced to mass spectrometry (MS) instrumentation (5). Although multiple SPME-MS couplings have been developed over the last 20 years, one in particular – coated blade spray (CBS) – has taken full advantage of the most recent advances in SPME technology to maintain the traditional operational simplicity of SPME while providing outstanding quantitation performance and speed of analysis (6). Here, I recapitulate my experiences under the supervision of Janusz Pawliszyn in developing CBS, as well as its suitability as a tool for solving some of the aforementioned challenges.
The solution
The story begins in 2013, when Pawliszyn assigned me to work on developing a rapid-diagnosis tool based on the direct interface of SPME to MS. Earlier that year, I had read about attempts to directly generate ions from SPME fibers by Pawliszyn (7)(8) and Jentaie Shiea (9). A few weeks later, while attending Pittcon 2013 in Philadelphia, I listened to a talk by Graham Cooks about two substrate spray technologies that were suitable for collecting small amounts of complex matrices (for example, blood and tissue). As Cooks explained, these technologies required no modification to the substrate and could perform electrospray ionization from one of their vertices with the assistance of a solvent (10). Thinking over this presentation – and aware of contributions by other outstanding researchers (7)(9)(11)(12) – it occurred to me that the substrate-spray concept (also described by Nobel Laureate John Fenn in one his patents in 1998, [11]) could potentially be coupled with the microextraction devices developed by the Pawliszyn group, particularly the blade geometry with a matrix-compatible coating. I was interested to find out if combining these technologies could produce something suitable for the rapid, clean, sensitive, and effortless determination of target analytes in complex matrices using MS. Anatomically, CBS is a sword-like device made of stainless steel that is partially coated with a matrix-compatible extraction phase (see Figure 1). The extraction phase can either be a polymer or polymeric particles (for example, hydrophilic–lipophilic-balance or octadecyl silica particles) that have been affixed to the substrate surface using a biocompatible binding agent. Analytically, the CBS workflow typically consists of three steps. First, the analytes are rapidly enriched on the coating (≤ 5 min), either by spotting the sample onto the CBS, or by extraction from a vial/well containing the sample. Second, the CBS is rapidly rinsed with water to remove any matrix components that may have adhered onto the coated surface during the extraction process. Finally, instrumental analysis is performed by applying a few µL of an elution/electrospray solution onto the coated area of the CBS. After few seconds (~ 20s), a high voltage is applied to the non-coated area of the blade, which results in the generation of ESI from its tip. The ESI process generates a transient signal that can be used for qualitative or quantitative purposes; the duration of the signal can be as short as 1s, but it can be increased to as much as a few minutes, depending on the application needs (for instance, when performing multiple MS events).Beyond the solution
Ideas rarely develop as quickly or easily as we would like, and my attempts to develop CBS were no exception. In 2013, our group had limited experience with ambient ionization and modifying MS systems, and this posed a significant challenge. Therefore, in the months following Pittcon, I worked tirelessly on the first CBS prototypes. I was eventually able to construct an MS-interface that was both suitable for this technology and safe for our instruments, and the resultant experimental data was encouraging enough to make it an idea worth pursuing. These early experiments demonstrated that achieving electrospray from a flat surface, such as a blade, was simpler than it was from a fiber, as was originally proposed in 2005. The metal substrate was able to produce stable electrospray by generating a constant electric field, and the thin matrix-compatible coatings facilitated a clean extraction mixture that was both rapidly desorbed into the electrospray solvent and efficiently introduced into the mass spectrometer. One year after the idea was conceived, our first results were published in Angewandte Chemie (6). In that manuscript, we demonstrated that CBS was capable of quantifying sub-ppb levels of cocaine and diazepam in urine and plasma without using chromatography or any other instrumentation aside from the MS system and the CBS. Furthermore, the analyte enrichment step could be performed in less than 1 minute, with a total analysis time of less than 3 minutes. Certainly, these features were revolutionary, especially for the most assiduous SPME skeptics who frequently pointed to its “long extraction times” and the “higher limits of detection” when compared with traditional SPE. Moreover, our results attracted the attention of Bradley Hart from Thermo Fisher Scientific, who has since been one of CBS’s biggest supporters. Despite our initial successes, we soon realized that there were several features that needed to be improved if CBS was ever to achieve commercial success. For instance, the first blades were coated using a spray mechanism that was both tedious and wasteful. Furthermore, the original blades were handmade, which made it difficult to reproduce the process. Fortunately, while my colleagues at the University of Waterloo and I were assessing novel methods for manufacturing CBS devices, we discovered a methodology that would allow us to homogeneously coat surfaces with a thin layer of particles (Ø≤ 15 µm). The introduction of thin, reproducible coatings enhanced the performance of CBS and speed of analysis, as it ensured better inter-device reproducibility, faster extraction, enrichment, and desorption of the target analytes; and minimal matrix component adherence on the coated surfaces. These improvements were essential in putting us back on the road towards successfully introducing our technology. We’re grateful to Mike Morris from Waters Corporation for providing 5µm particles, which facilitated very thin and robust coatings. Predictably, criticism of our CBS technology emerged shortly after our initial results were published, with some authors quick to claim that it could not be applied to the analysis of spot-sized sample volumes. To refute this, we demonstrated that CBS could rapidly quantify a drug with a narrow therapeutic range (amitriptyline) from blood droplets (≤ 10 µL) (13). Furthermore, in a subsequent publication, we also revealed that the results obtained by either methodology (spot analysis or extraction from a vial) were comparable, and that the total instrumental time could be reduced to just a few seconds without compromising the technique’s quantitation capabilities (14).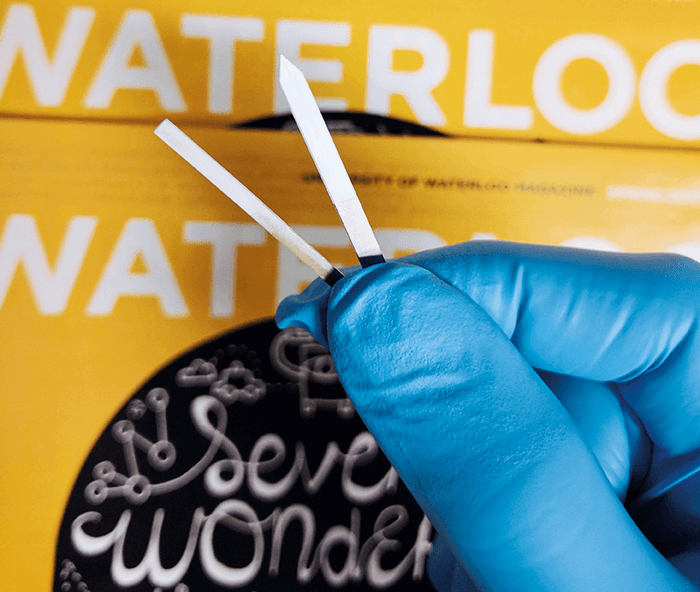
Aware of the need for speed, accuracy, and unmanned operations in clinical laboratories, we demonstrated that, like the traditional blade geometry, 96-CBS devices could be arranged in a holder to perform high-throughput automated extractions from a plate containing an equal number of samples, thus reducing the sample preparation time to less than 7 seconds per sample (15). Furthermore, we showed that CBS was suitable for the rapid screening and quantitation of at least 18 substances with different physicochemical properties at concentrations typically monitored by international regulatory agencies (for example, the World Anti-Doping Agency) (15). Although the total instrumental time in this study was in the order of 20 seconds per sample (MS-time), it can be further reduced, allowing for total analysis times that are faster than any available on-line SPE technology. Moreover, given its structure, CBS can be used to perform analyte enrichment from diverse biological and food matrices without having to worry about cartridge clogging, carryover, or costly repairs in pumping systems. At present, we are focusing on achieving the complete automation of the CBS workflow. Such a system would potentially allow CBS to attain total analysis times of under 10 seconds per sample when monitoring 20 to 30 target compounds – even faster analysis can be achieved for a smaller number of targets or when performing molecular profiling studies. Since its inception, we have held a firm belief that CBS will be a paradigm-shifting technique not only in the clinical field, but also in the fields of environmental, forensic, and food analysis (16)(17). Its potential applications range from determining pesticides or chemical warfare agents in drinking water, to classifying food samples from different farming origins, to quantitating biomarkers on a droplet of plasma, to name just a few. We envisage CBS as an analytical tool that can radically alter how we go about direct sample introduction to MS. Yet, in the words of AC/DC, “It’s a long way to the top!” and CBS is still working towards widespread acceptance by the scientific community and the industry. The good news is that the US Patent and Trademark Office has recently granted a patent for CBS (18), and we are now teaming up with Restek, one of the world’s leading analytical science corporations, to bring this technology to interested users around the globe. Indeed, JP scientific, the company holding the patent rights for CBS, is open to partnering with any MS companies potentially interested on this technology. It is important to keep in mind that, whereas CBS is intended to be a tool for rapid screening and quantitation, thin-film coated blades without a sharp tip can be used for traditional confirmation experiments via LC-MS/MS; as such, there is ample opportunity for interested scientists to evaluate the performance of this technology using a more traditional determination format. We are eagerly looking forward to hearing about the various ways researchers apply this blade technology to overcome their challenges. German Augusto Gómez-Ríos is now a postdoctoral fellow in the Pawliszyn lab, Department of Chemistry, University of Waterloo, Canada.
References
- RP Belardi, JB Pawliszyn, Water Pollut Res J Can, 24, 179–191 (1989). ÉA Souza-Silva et al., TrAC Trends Anal Chem, 71, 224–235 (2015). EA Souza-Silva et al., TrAC Trends Anal Chem, 71, 249–264 (2015). D Vuckovic et al., Anal Chem, 80, 6870–6880 (2008). L Fang et al., TrAC Trends Anal Chem, 85, 61–72 (2016). GA Gómez-Ríos, J Pawliszyn, Angew Chemie, 53, 14503–14507 (2014). J Pawliszyn, US Patent 7384794 (2003). M Walles et al., Chromatogr A, 1067, 197–205 (2005). CP Kuo, J Shiea, Anal Chem, 71, 4413–4417 (1999). CR Ferreira et al., Clin. Chem, 62, 99–110 (2015). J Fenn, US Patent 6297499B1 (1998). F-L Hsu et al., Anal Chem, 75, 2493–2498 (2003). H Piri-Moghadam et al., Angew Chemie, 55, 7510–7514 (2016). M Tascon et al., J Pharm Biomed Anal, 10.1016/j.jpba.2017.03.009 (2017). M Tascon et al., J Anal Chem, 89, 8421–8428 (2017). J Poole et al., Environ Sci Technol, 10.1021/acs.est.7b03867 (2017). GA Gómez-Ríos et al., Anal Chim Acta, in press (2017). J Pawliszyn, GA Gómez-Ríos. US Patent 9733234 (2015).




