Photo provided by Nicholas Drachman
A novel nanopore ion source developed by researchers at Brown University directly delivers ions of amino acids and peptides into high vacuum, circumventing the typical sample losses associated with conventional electrospray ionization (ESI) in mass spectrometry. The new ion source shows promise for advancing proteomics by improving ion transmission efficiency.
Mass spectrometry relies on ion sources such as ESI to generate vapor-phase ions from liquid samples. Traditional ESI involves creating charged droplets, but the necessity of a background gas to evaporate these droplets results in substantial sample loss before the analyte reaches the mass analyzer. In contrast, the nanopore ion source developed in this study avoids such losses by emitting ions directly into the vacuum environment, resulting in a substantial boost in ion transmission efficiency. The researchers demonstrated up to a two-order-of-magnitude improvement over traditional ESI methods.
Their inspiration for the nanopore ion source was twofold: “First was the success nanopores have enjoyed in the arena of DNA sequencing; second was a simple calculation of how small an electrospray ion source would have to be to produce droplets that could not contain more than a single ion,” say co-authors Derek Stein, Professor, and Nick Drachman, PhD student, who both work in the Physics Department at Brown. “It became clear that an ion source should behave differently, and possibly in useful ways, if the orifice shrinks to a small but still manufacturable scale.”
The researchers started by trying to emit ions from a nanoscale hole in a flat membrane – because they knew how to manufacture those. “The results were disastrous. There were electrical arcs and sparks in our vacuum chamber as the membranes disintegrated,” say Stein and Drachman. The researchers learned their lesson and shifted to making nanoscale openings at the tips of capillaries. “One eureka moment was when we discovered that such an ion source emits ions directly into the mass spectrometer,” they say. “A related eureka moment was when mass spectrometry measurements revealed that no liquid droplets were produced at all.”
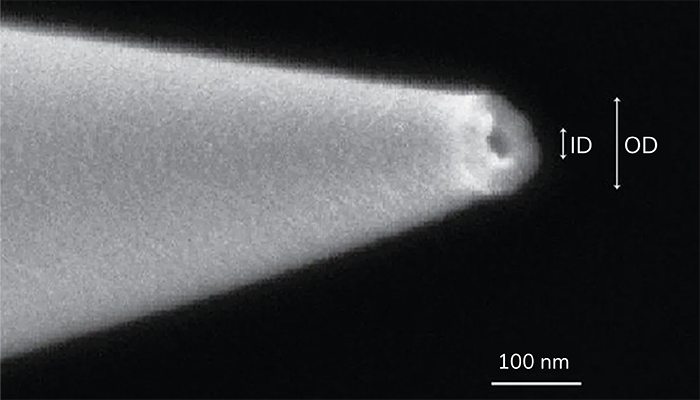
The heart of the system is a quartz nanopipette with a sub-100 nm opening, through which amino acid and peptide ions escape into the vacuum. By applying an electric field to the meniscus of the liquid sample at the pipette tip, ions are emitted via ion evaporation. This method allows the source to bypass the charged droplet stage, a key feature of ESI, and directly transfer ions into the mass spectrometer without the need for a background gas.
The research team tested the ion source using a custom-built quadrupole mass spectrometer. They successfully detected 16 different amino acid ions, including post-translationally modified peptides, and were able to produce mass spectra of these analytes in just seconds. Notably, more than 90 percent of the emitted current from the ion source was recovered at the detector, underscoring the source’s high efficiency.
The study highlights the ion source’s ability to emit desolvated ions rather than charged droplets, which simplifies the analysis by reducing the noise and signal broadening that typically occur in ESI. The researchers hope to eventually sequence a protein by measuring every amino acid by mass spectrometry. "We think that the emission of unsolvated ions reduces the ambiguity in identifying a particular ion," they say. Additionally, the system shows promise for single-cell proteomics – which necessarily deals with small samples, and the proteins in those samples cannot be amplified like nucleic acids can.
“Therefore, we need to avoid sample loss as much as possible to maximize proteome coverage,” say Stein and Drachman. “At present, the highest sensitivity is achieved with nano-electrospray ionization sources, but even they typically lose about 99 percent of analyte ions. The nanopore ion source circumvents the main loss mechanism by emitting ions directly into the high-vacuum part of the mass spectrometer.”
However, the overarching goal of the research was single-molecule sequencing. “Mass spectrometry ticks many of the boxes that a sequencing technology should: high contrast between different monomers, single-ion sensitivity, and high bandwidth,” say Stein and Drachman. “Nevertheless, we don’t usually think of mass spectrometry as being a single molecule technique – but it is up to the challenge. What has been missing is a technique for reliably transferring single ions into the mass spectrometer with high efficiency. The nanopore ion source solves that problem.”
The researchers also explored the pH dependence of amino acid ion emission rates, noting significant variations in the emission of certain amino acids like histidine and arginine. This study provides further insights into the ion evaporation mechanism and its efficiency under different conditions.
Going forward, the researchers are extending the research along two lines. “First, we are working on ways of using light to fragment a protein into separate amino acids while it is still in the liquid inside the ion source,” say Stein and Drachman. “This is the next step on the roadmap of our single-molecule protein sequencing concept. Secondly, we are redesigning the nanopore ion source so that it can be tested and used with commercial mass spectrometry instruments. We see this as an important step toward commercialization.”




