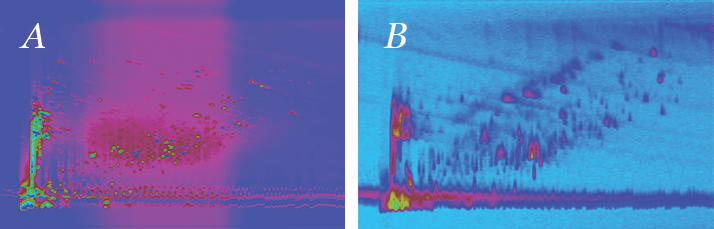
Chinese medicine is an ancient art. The earliest existing Chinese text on medicine dates back to the second or first century BC – the Huang Di Nei Jing. Typically, in early Chinese medicine, several plants, each with hundreds or even thousands of compounds, are formed into a single drug formulation. Today, much has remained the same – the normal formulation of a Chinese herbal medicine (CHM) is extremely complex. Product control that is comparable to Western medicine is a huge challenge. CHM has, unsurprisingly, attracted a lot of attention throughout the world as our search for more effective medicines delves ever deeper. It follows that researchers are interested in the chemical constituents of Chinese herbs and the origins of their pharmacological and thus therapeutic activities. Essential separations We’ve probably all heard someone say that because the number of labs with high-resolution mass spectrometry (HRMS) is increasing, less and less chromatography will be required ahead of detection. For the non-chromatographers out there, that might even sound quite tempting. However, in reality, it’s a statement that does not really make much sense: if all components of a sample are injected into the ion source at the same time and if a large percentage of those compounds are ionized, then – in a complex sample like Chinese herbs – several thousands of radical cations will be formed. And in an atmospheric-pressure ion source, such as ESI or APCI, all of these resulting radical cations can react or interact, each encountering approximately 20,000 collisions from the point of ionization to the entrance of the MS. The result is potential ion suppression and/or formation of artefacts. That’s a problem. The solution? The addition of a high performance chromatographic platform in front of the MS! Such a combination is (and may always be) the gold standard, even with HRMS. Comprehensive 2D-LC – or LCxLC – offers the high performance separation desirable for complex samples. Indeed, our group specialises in the use of comprehensive techniques to analyze various herbs in CHM. More non-polar species are analyzed using GCxGC-MS, whereas more polar compounds are analyzed using LCxLC-MS (see Figure 1).
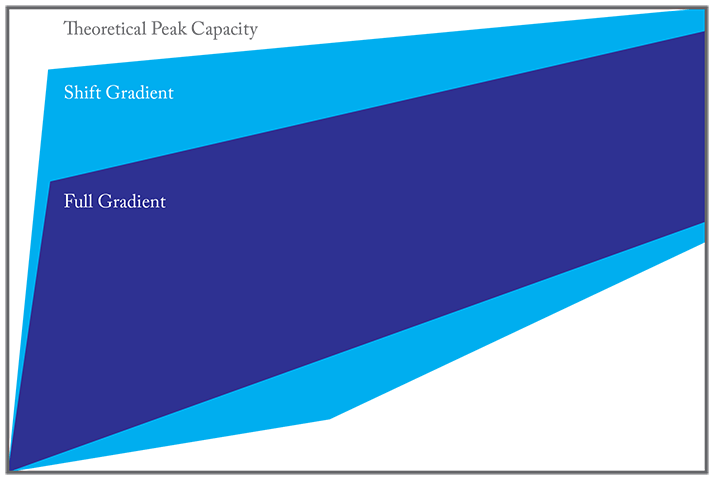
Of course, as with any analytical technique, there are advantages and disadvantages of LCxLC, which are dependent on the application. For us, when compared with one-dimensional LC, the disadvantages of LCxLC could be summed up as potentially lower sensitivity with MS-detection and the more complex method development (1). However, the advantages are clear: much higher peak capacity (as noted in the first article of this series: tas.txp.to/0314/2DLC) and the ability to produce contour plots that display peak intensity as a function of the retention times in the first and second dimensions – these are excellent for fingerprint-style analysis. A shift in gear So, how can we further optimize LCxLC separations? The answer is in the gradient programs used (see Figure 2). Our system allows the use of a constantly shifted gradient in the second dimension, which uses a narrower range of mobile phase composition than the full gradient program but continuously shifts the concentration range according to retention. The shift gradient is really a combination of a parallel gradient and a full gradient; the lower concentration range enables the retention of weakly retained fractions, while the higher concentration range is sufficient to elute strongly retained fractions, as with a parallel gradient. The shift gradient offers bandwidth suppression but also reduces the probability of “wrap-around” behavior, just like a full gradient.
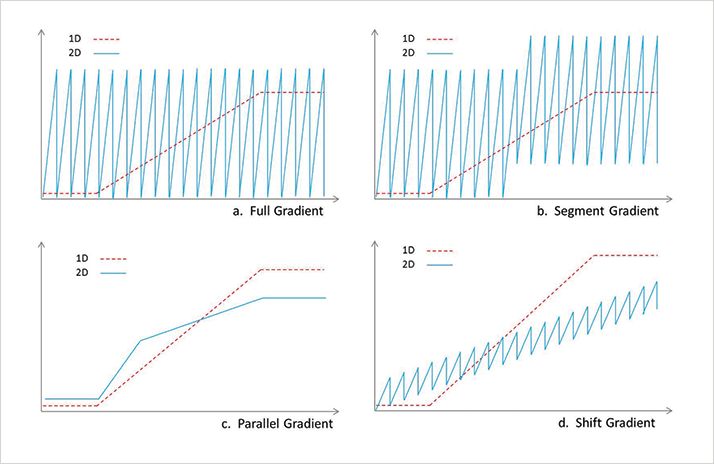
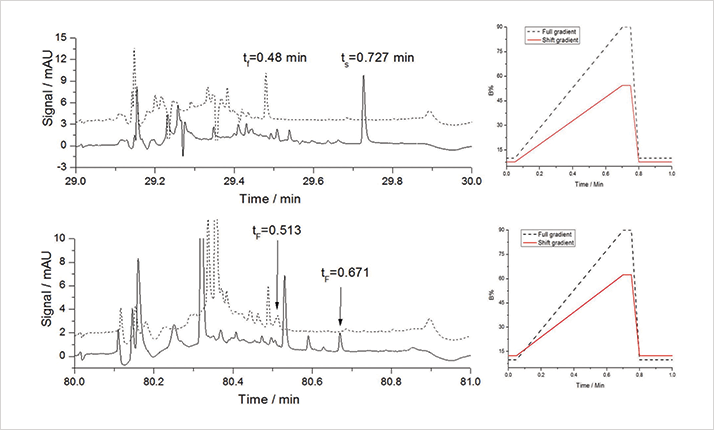
In a correlated RPLCxRPLC system, the early-eluted analytes in the first dimension will have a weak retention in the second dimension; the analytes eluted in the middle of the first separation will be eluted in the middle of second dimension; and the late-eluted analytes in the first dimension will have a strong retention in the second dimension. And because the shift gradient runs in a continuous way, the cluster information in real world samples is preserved. Undoubtedly, the shift gradient increases the separation power in the second dimension significantly as shown in Figures 3 and 4 (2).
2D-LC or not 2D-LC, that is the question
Should you be leveraging the power of 2D-LC? If you want increased separation of complex samples, then absolutely! Non-targeted analyses, such as identification of disease biomarkers, have become much more powerful using LCxLC-MS. And we are only at the beginning. The next step is LCxLC-IMS-qTOF-MS. Some of the first investigations of this sort are being done in my lab right now…
Next month, the “Demystifying 2D-LC” series will tackle biopharmaceutical analysis.
References
- A. P. de la Mata and J. J. Harynuk, “Limits of Detection and Quantification in Comprehensive Multidimensional Separations”, Anal. Chem. 84, 6646-6653 (2012). D. Li and O. J. Schmitz, “Use of Shift Gradient in the Second Dimension to Improve the Separation Space in Comprehensive Two-dimensional Liquid Chromatography”, Anal. Bioanal. Chem. 405, 6511-6517 (2013).