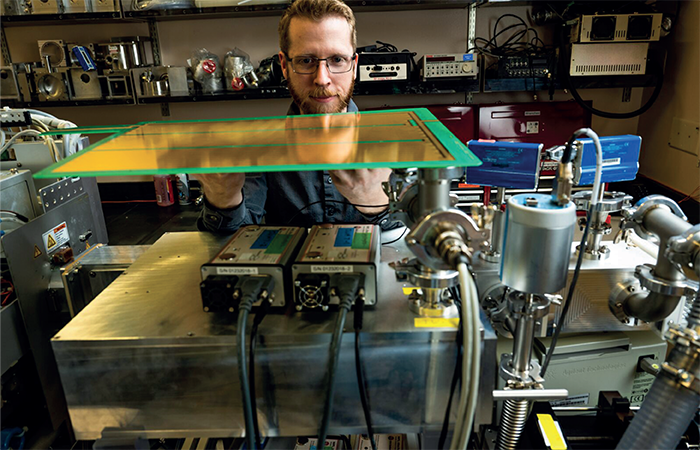
Despite all the scientific advancements over the years, we still only know the complete chemical structure of around 5 percent of all the molecules we believe to exist. This number is staggeringly low and needs improving so that we can learn more about how molecules react within the environment – and what role they play in biological pathways. With this in mind, my team and I decided to create a new instrument to improve the identification rate of unknown molecules.
Many compounds have similar structures, which makes identification complicated. This is especially true for complex biological mixtures. Another common issue is that many unknown compounds only exist in low concentrations, and this means we might not even see them, much less identify them. All these issues must be considered when trying to identify unknown molecular structures.
Two common approaches used by researchers to identify unknown molecules are nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry (MS). NMR is considered a definitive structural measurement, but a fairly pure concentration sample must be obtained for it to be used. On the other hand, MS is widely used to analyze mixtures, but it doesn’t typically provide enough information to definitively determine a chemical structure – especially when used alone. Other gas-phase analysis techniques, such as infrared (IR) spectroscopy, ultraviolet photodissociation (UVPD), and ion mobility (IM) spectrometry can be paired with MS.
However, we believe that the combination of high-resolution MS and high-resolution IM spectrometry shows tremendous promise in identifying the chemical structures of unknown ions. And that is what the SLIM-Orbitrap is designed to do.
To use the SLIM-Orbitrap, we first turn molecules into ions (molecules with an electrical charge) – making certain measurements easier on ions than it is on molecules. We currently use electrospray ionization (ESI) to do this. Once ions are created, they enter the SLIM-Orbitrap.
The Orbitrap mass spectrometer was developed by Thermo Fisher Scientific to measure an ion’s mass electric charge and how the ion breaks apart – providing insights into an ion’s constituent atoms, and helping us determine the ion’s chemical formula. The second instrument used in our study is known as SLIM: structures for lossless ion manipulations – which is an ion mobility spectrometer that measures the size and electric charge of ions. SLIM addresses the challenge of differentiating ions with identical mass and fragmentation patterns, but differing atomic bonding (structural isomers).
We cannot measure an ion’s mass with this instrument alone, which is why we combined SLIM with the Orbitrap for a more detailed analysis. The combination of these two instruments allows us to measure multiple different properties of ions with very fine details, and we use all these fine details to help us determine the structure of an unknown ion.
To couple these machines together, we used the dual-gate scanning technique, which involves pulsing a small number of ions into the ion mobility spectrometer and letting the ions separate. Instead of releasing all the ions at once, only a few at a time are allowed to exit the instrument based on their arrival speed. Two ion gates are used to make a “dual-gate” setup, and “scanning” the second ion gate allows us to send ions with slightly lower speeds to the mass spectrometer until all the ions are analyzed. The dual-gate scanning technique allows us to operate both instruments at their full potential.
There are a few known disadvantages to this technique (for example, sample wastage and long experiment times) but, overall, SLIM-Orbitrap provides us with high-resolution results that effectively distinguish and analyze ions with high precision.

Our main findings revealed that a combination of high-resolution SLIM and Orbitrap measurements can assist in distinguishing ions that cannot be achieved with other techniques. One instance of this was shown through the analysis of a lipid mixture – the Orbitrap couldn’t tell the difference between two lipid structural isomers, but the SLIM instrument could. Though we couldn’t fully tell what these two lipids were, the Orbitrap identified them as phosphatidylcholines, and SLIM revealed that they were slightly different in size.
Though these results are exciting, the system is quite new and further work is required. We’ve already explored compounds in many samples (such as lipids, dyes, and metabolites), but we want to push this machine to its full potential. These systems can analyze almost every ion that you put into them – expanding our research capabilities even further.
Moving forward, we’re hoping to use the SLIM-Orbitrap to fully identify unknown compounds that challenge other researchers. And though we aren’t yet sure on the role of AI, computational models are sure to play a big role in decoding the data acquired from the SLIM-Orbitrap.
SLIM-Orbitrap is part of the m/q Initiative – a five-year investment at Pacific Northwest National Laboratory to develop a predictive understanding of unknown compounds through MS. The current standard approach of synthesizing and comparing chemical standards is time-consuming and costly. Predicting unknown ion structures based on many different high-resolution measurements could revolutionize sample analysis and save time, effort, and money. We believe that the SLIM-Orbitrap is part of the solution to achieving this goal.
Some researchers remain hesitant to adopt relatively new technology, such as ion mobility, because their workflow has been established for a long time. However, we hope that the SLIM-Orbitrap shows what’s possible with new and emerging technologies, like high-resolution ion mobility, and just a little investment. The big takeaway from this study? The current model needs improving if we’re to use MS to its full potential. With further research, we expect to see very exciting discoveries in the not so distant future.