Whole column imaging detection (WCID) has revolutionized charge-based protein separation with capillary isoelectric focusing (CIEF) providing rapid method development, high resolution, reduced sample volume, and high-throughput analyses. But how can these advances be reliably coupled to mass spectrometric detection?

Biopharma and life science protein researchers need high-resolution, high-sensitivity, and high-throughput protein separation tools for product development and quality control; they need to know the nature of their target isomers and they may need to provide structural information. Mass spectrometry (MS) is the best approach for protein structure characterization because it facilitates understanding of the nature of protein isomers, posttranslational modifications, and product degradation pathways. I became passionate about WCID with CIEF while I was a graduate student at the University of Waterloo in Canada; it was here where the concept of WCID was conceived and developed. That was in 1998, when we had to build a WCID CE system with a high voltage power supply, a CCD camera, and whatever cartridge we could assemble. Though this may seem simple to do, it was very challenging because we wanted to generate high quality, reproducible results. I found, as other researchers did, that there were many fundamental things that needed to be addressed before CIEF could be used as a reliable and robust analytical tool for protein separation and characterization. After graduating, I joined Convergent Bioscience Ltd (Toronto, Canada), a startup company that was a pioneer in commercializing WCID CE technology. I wanted to improve the detection sensitivity of WCID, to develop more (and improved) reagents and test kits so that WCID could have broader applications. However, at the time WCID technology could not provide efficient fraction collection for MS protein isomer characterization nor could it be coupled directly to a MS. The problem was that the inner and outer diameter (ID; OD) of the separation and transfer capillary that connect through a small section of membrane capillary were the same. The ID of the membrane capillary, however, was larger than the OD of the separation and transfer capillary – this gap caused a loss in resolution when forcing the focused proteins from the separation capillary into the transfer capillary. Also, separated proteins remixed in the large ID section of the hollow capillary membrane. So, researchers had to use other fractionation methods such as liquid-phase isoelectric focusing to isolate protein isomers before they could be characterized.
We wanted a solution, and none seemed forthcoming, so it was time to take matters into our own hands. But developing a state-of-the-art analytical instrument is not simple; you need highly qualified mechanical, electrical, software, and production engineers. AES really all started from trust, a willingness to collaborate closely, and an eye for evaluation. And it took us several years to select and form such a team with the necessary diversity in background. I firmly believe that the combination of great chemistry, openness, and trust among team members has been the foundation for our solution. In brief, we have developed what we believe to be the world’s first high-resolution iCIEF fractionation and direct-coupling iCIEF to MS solution, which comprises the CEInfinite WCID CE instrument, MS compatible capillary cartridges, and related test kits. We have also developed our own carrier ampholytes and stable capillary coatings, which are essential for protein CIEF. Because we develop and manufacture all the key components in house, we can customize reagents to better suit diverse protein sample separation challenges. The CEInfinite system is based on WCID and uses a small length of separation capillary (from 2 to 10 cm) in a column-diameter transformation cartridge (patent pending), which is monitored by an imaging sensor (see Figure 1). Detection is provided by a series of snapshots of the whole separation capillary. The advantage of WCID over conventional single point detection is real-time monitoring, which allows high throughput CE separation and study of dynamic separation processes.
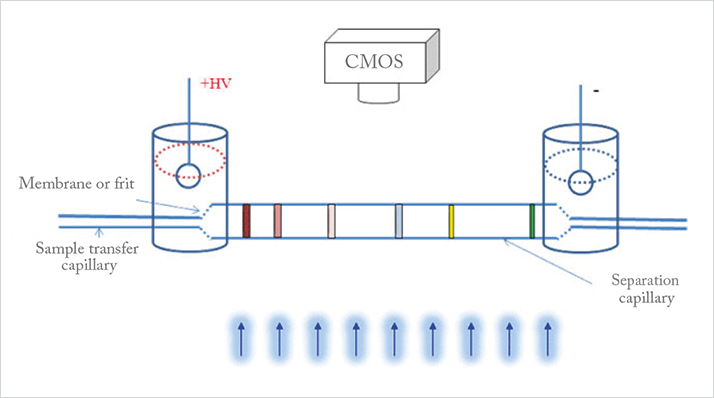
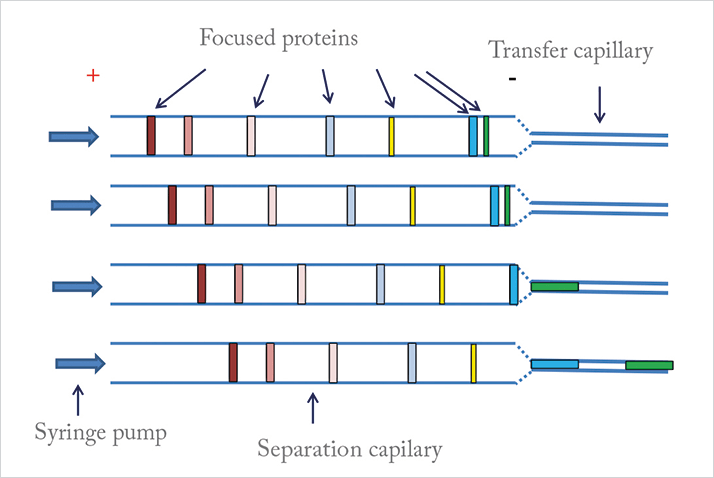
In fact, the cartridge is at the heart of the system and comprises a large ID separation capillary, and a small ID transfer capillary. The larger ID of the separation capillary improves the detection sensitivity, but once the proteins are separated, they move into the transfer capillary with minimal peak remixing ahead of preparative fractionation or direct coupling to the MS system (see Figure 2). Though there has been an increasing demand for high sensitivity detection and CIEF-MS for many years, no one had really been able to find an answer. Indeed, it took a great deal of research and tests back and forth, before we found the answer in unconventional column-diameters. But the solution isn’t an easy one; the manufacturing of such unconventional cartridges with small capillaries of different IDs is very difficult. Nevertheless, our chemical engineering team has worked hard to create a proper formulation for the membrane, and our production team is now able to consistently manufacture cartridges according to our stringent criteria. And so more recently, we have been very focused on demonstrating the high-resolution protein isomer fractionation of our cartridges, and in the process, we’ve seen unexpected CIEF separation improvements for some complicated fusion proteins when using our larger ID (180 or 200 μm) cartridges. Notably, the carrier ampholytes (Aeslytes) also play an important role by helping to improve resolving power for other fusion proteins and antibody drug conjugates. From a practical point of view, the cartridges also facilitate sample injection and reduce capillary clogging. In summary, a number of features have been implemented together to create our solution for CIEF-MS:
- The ID of the separation capillary is much larger than that of the transfer capillary; therefore, remixing of separated protein during transfer after CIEF is minimized.
- MS compatible cartridges allow protein fractionation or coupling to MS after CIEF, which enables protein isomer structure characterization with WCID, something that has never been achieved before.
MS compatible carrier ampholytes (Aeslytes) minimize interference of ionization of protein, during mass spectrometric detection. - Small molecule pI markers consist of non-peptide synthetic molecules that cover the full pH range from 2.5 to 10.5, allowing convenient application of pI markers even in the presence of enzymes, such as carboxypeptidase B.
- The ID of the separation capillary is a major factor which determines the detection sensitivity for WCID CIEF, which is why our cartridge technology is so important.
So far, we have sold systems and a variety of CIEF related products to biopharmaceutical companies, research institutions, and universities in North America and Asia. We have also just launched our full product line in Europe. In the next few years, we aim to become a leading total solution provider for high-throughput and high-resolution protein separation and characterization. To do this, we are working closely with researchers in the biopharma industry and life science research institutions to pinpoint their needs and provide further solutions. I foresee that our products will be desirable in therapeutic protein development and quality control in the biopharma industry, as well as in clinical laboratories for protein disease marker analysis – so we are keen to develop strong collaborative relationships in these areas. As with any new system, support is important, and our team of experts can aid in method development (which can take only a couple hours for simple protein and up to a week or so for complicated samples). Our ultimate hope is that by applying our technology, with its high analytical throughput and capability to perform multiple assays (for instance, protein identity, protein binding, and protein concentration), our clients can speed up the drug development process – and we are passionate about trying to help our customers in this endeavor. I have to say that a personal highlight for me was when we received an evaluation from a major potential customer, with a glowing report of our CEInfinite system in routine sample analysis. In the extensive four-month validation process, many therapeutic proteins were analyzed, and we were informed that our CE system provided better reliability and reproducibility, with separation and quantitation results comparable to other CE instruments that they had used. Our CE system also provided better resolution for some monoclonal antibodies and fusion proteins. And of course, we have found the missing link between iCIEF and mass spectrometry. We are proud of what we have achieved up to this point – and we look forward to developing our WCID technology further.




