Michael is currently a senior research scientist with Eli Lilly and Co, Indianapolis, Indiana, USA). His laboratory works on top down proteomics with high resolution mass spectrometry, informatics for proteomics, identification of protein modifications, protein chemistry, and biomarker, and drug target discovery and validation. He currently has three CE-MS systems in his lab and is working on improvements for the technology.

Norm is the Grace-Rupley Professor of Chemistry and Biochemistry, at the University of Notre Dame, Indiana, USA. His postdoctoral fellowship at Los Alamos Scientific Lab introduced the concept of single molecule detection, leading to the development of a capillary array DNA sequencer that became the workhorse tool used in the human genome project.

“Although I apply several other technologies in my research, CE-MS is my main approach. It often provides information that cannot be obtained with any other technology,” says Rob, senior post-doctoral researcher at the VU University Amsterdam, where his research is directed at characterizing biomacromolecular compounds. CE-MS has undergone positive development over the last few years, he explains. “CE-MS was mainly used by academics, but today I am in regular contact with companies that are starting to use (or are planning to use) CE-MS in their laboratories. I am able to share my knowledge with them and in return I get a good insight of the research questions that drive them.”

Tomoyoshi majored in applied chemistry and graduated from Keio University, Tokyo, Japan. He then spent 17 years as an application chemist for HPLC and CE at Yokogawa Corp. and Yokogawa Analytical Systems Inc. He is a pioneer in the development of CE-MS based metabolome analysis technologies. His research interests include development of CE-MS methods for metabolome analysis, understanding underlying mechanisms for the regulations of cancer cell metabolism, and biomarker discovery.

Christian is an R&D scientist for Agilent Technologies in Waldbronn, Germany. He joined Agilent Technologies in 2002, where he became heavily involved in instrument and application development for automated electrophoresis systems (for example, the 2100 Bioanalyzer and the 2200 TapeStation). Since 2009, he has worked with the 7100 CE system and has authored several application notes and other technical literature for the instrument.

ND: CE has had one overwhelming success – the sequencing of the human genome (1)! And though I believe the technique has matured in terms of theory and technology, detector sensitivity continues to be its main weakness.
MK: For the biopharmaceutical industry, CE is an excellent substitute for polyacrylamide gel electrophoresis (PAGE) based techniques in protein size and isoelectric point determinations. Such applications are robust and are available in kits from vendors. But despite the many publications on coatings and separation methods for CE analysis, it is still not widely used by analytical laboratories.
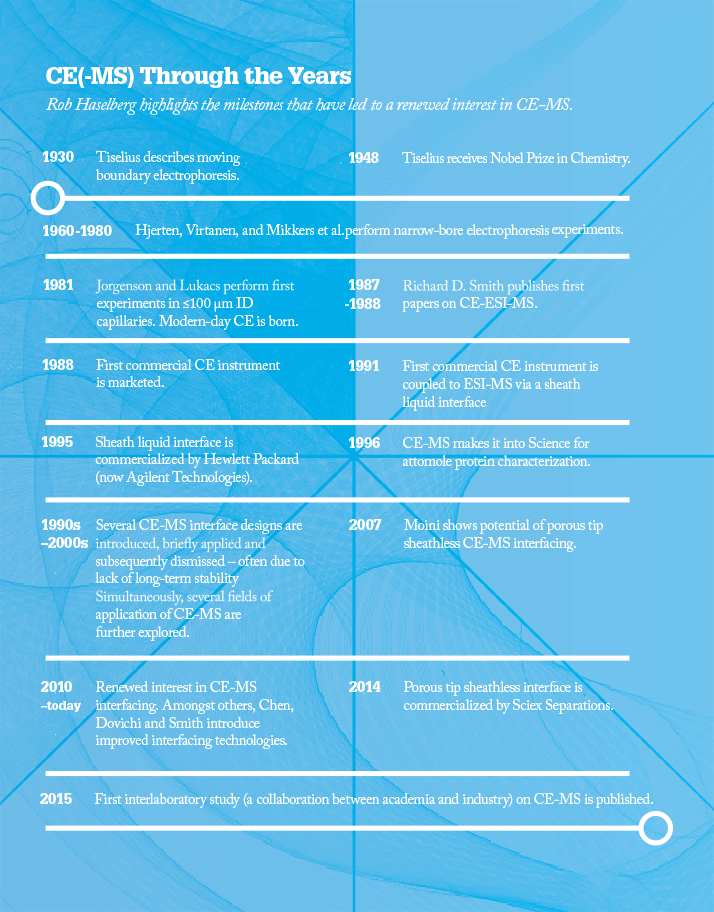
RH: It is well established in many fields: Norm has already mentioned genomics, for example, and then there’s biopharmaceutical characterization (see Figure 1). CE is also established in glycan and ion analysis, and chiral separations. In the early days of CE, Stellan Hjertén and later Jim Jorgenson played an important role in its development. Since then, many other people have contributed to its success – Dick Smith, Barry Karger, Andras Guttman, Norm (of course), and Jonathan Sweedler to name but a few. They’re all responsible for pushing the technology into different fields. The main challenge for CE has (unfairly) been the high expectations set in 1980s and 1990s, which it didn’t live up to; today, the technology still struggles to shake off the negativity because of those early failings.
TS: Back in the late 1990s, I worked as an application chemist at Hewlett-Packard where I developed several CE applications, such as a simultaneous anion analysis method. Many Japanese electroplating bath companies bought CE systems and kits for quality control. Also, food companies used the method. Nevertheless, sensitivity, identification capability and migration time stability were – and remain – problems for the technology.
CW: CE is now vital in biopharmaceutical R&D and production. Over recent years, methods such as CZE (capillary zone electrophoresis), cIEF (capillary isoelectric focusing), CE-SDS (CE- sodium dodecyl sulfate) have been adopted as specification methods for the majority of newly marketed biotechnology protein products. Many biopharmaceutical companies – of all sizes – have implemented these methods. Regulatory agencies have also recognized the value of CE methods and now encourage the shift from slab gel-based methods to CE. As well for other high molecular weight biomolecules like DNA or RNA, CE (using capillaries or microchips) is now a routine method, for example for sample quality control in next generation sequencing workflows. For the analysis of low-molecular-weight pharmaceuticals, CE has evolved into a prime separation tool; for example, for separating enantiomers, many other ionizable molecules of high biological relevance, and for ion analysis. Implementing robust methodologies, using rigorous protocols for capillary preparation and pre-conditioning, and automatic buffer replenishment has played an important part in developing CE. And, of course, conferences have helped educate people about its utility, encourage its use, and drive development. Close to maturity is UV-Vis detection in CE, which can achieve sensitivities close to theoretical limits and provide a large linear dynamic range by using extended light path capillaries or Z-shaped flow detection cells. However, CE is inherently a micro-separation technique that can’t be scaled up to larger dimensions.
RH: As with any separation technology hyphenated to MS, CE and MS is an extremely strong combination. Separation of mixtures decreases complexity prior to detection, so more (mainly low-abundant) compounds can be detected, and the coverage of sample constituents is much higher. CE is an orthogonal technique (compared with LC) when it comes to its separation mechanism. Different compound classes or types of modifications can, therefore, be separated, which will provide other important insights. Moreover, MS can provide the identification, sensitivity and selectivity often needed in contemporary analysis.
ND: Mass spectrometry coupled with CZE (note the Z) is now generating interesting applications in both proteomic and metabolomics analysis. These applications arise from improved interfaces and mass spectrometers, which are producing quite high detection sensitivity.
MK: Today, CE is in a similar place to where HPLC was before it was linked to a mass spectrometer, where retention time was the only measure of peak identity compared to a standard. When an HPLC system was eventually connected to a mass spectrometer, peak identity was determined by retention time, mass, and fragmentation. Crucially, the sensitivity of modern mass spectrometers can overcome the main limitation of low injection volumes on CE separation, allowing for sufficient MS signal acquisition. Now, CE peaks can have a true physical mass measurement and an additional orthogonal separation for coeluting species when connected to a mass spectrometer.
CW: Hyphenation enables compound confirmation and identification with high specificity. CE-MS has proved to be excellent for protein and peptides (for example, hydrophilic peptides and glycopeptides). Also, native and intact proteins, or conjugates, can be analyzed with virtually no upper molecular weight size limit, a task that is difficult to accomplish with LC-MS.
RH: In research, CE-MS is definitely an established technology. I am aware of labs, such as those of Tomoyoshi and Harald Mischak, where CE-MS is routinely used in bioanalysis of metabolites and peptides. Clearly, this is possible when proper training is provided. In other industries, it is used increasingly in R&D. However, there is not much use of MS in general by QC-type laboratories – those people prefer optical detection. Therefore, it is not surprising that in a QC environment you won’t see much use of CE-MS.
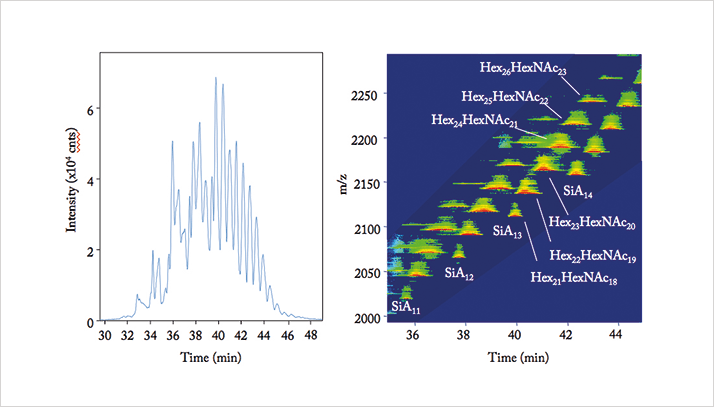
TS: In 2003, I first developed a metabolome analysis method based on CE-MS, which enabled the simultaneous analysis of several thousand charged metabolites by cationic and anionic methods (for examples, see Figure 2, 3 and 4). Since then, I believe that CE-MS has become one of the standard methods in bioscience research.
MK: Currently, research laboratories are working out the sample handling and robustness issues. And, it is in use in metabolomics laboratories. However, it is a challenge to find CE methods that are compatible with mass spectrometry. I don’t think that CE-MS is a mature standard platform for analysis, but it is an area that is ready for growth as current challenges are overcome.
ND: CZE-MS is awaking from a 20-year slumber and it is attracting modest interest from the research community. Its main applications are found in biopharma for analyzing recombinant therapeutics and characterization of glycans. Secondary applications may come in clinical analysis of peptides.
CW: Besides GC-MS and LC-MS, CE-MS is a key technology in metabolomics. And in proteomics, it is not only a complementary technique, it is also a true alternative to complex nano LC-MS. For biopharmaceuticals, the determination of charge heterogeneity and glycosylation by CE-MS has the highest potential and it is getting a lot of use for those kinds of applications.
TS: I think there are many opportunities. For example, I founded a bio-venture, Human Metabolome Technologies (HMT), which utilizes CE-MS extensively and has been listed on the stock market since 2013. We perform contract metabolomics analysis for university and industry customers.
ND: The biopharma industry is beginning to pay attention to CZE. This interest is driven by regulatory agencies, who demand careful understanding of the structure of recombinant therapeutics and identification of host-cell (and other) impurities present in those therapeutics.
RH: I think industry is beginning to recognize the benefits of CE-MS. For example, many companies are interested in having CE-MS available in their research labs – mainly those involved in protein analysis, as CE can provide separations that are not easily achieved with any other technology.
MK: I would say there’s an opportunity for developing applications with the increase in sensitivity and robustness of new CE-MS interfaces – and I think industry is ready to accept it if it proves to be robust and commercially available. However, it must perform better than other existing methods if it is to gain wider acceptance.
CW: Equipment suppliers should look more into providing solutions rather than what users perceive to be black box technologies and methods. Another opportunity is electrokinetic sample preparation methods in CE as opposed to SPE in HPLC, which should be interesting, for example for the food industry.
RH: CE-MS is strong in areas where other technologies do not provide optimal separations. Protein analysis is something that can be done with CE-MS quite easily these days. Also, the analysis of small polar compounds is easier with CE-MS compared with other methodologies. It is in these areas where investing in CE-MS is well worth doing.
MK: CE is an easy way to separate proteins and analyze them in a mass spectrometer. So, intact protein analysis, top down proteomics applications and immunoprecipitation MS-based assays will benefit from it. It is the methods for CE-MS that deliver the superior separation ability that will drive the applications.
CW: The highest growth potential is in the biopharmaceutical industry, metabolomics, and proteomics.
ND: Again, CZE is well suited to characterization of recombinant therapeutics. And there have been interesting applications of CZE for characterization of urinary and spinal fluid peptidomes in clinical studies.
TS: Compounds that are low molecular weight charged species are well suited to CE-MS analysis and ideal application areas are bioscience, clinical, food and fermentation.
MK: Yes, I do. The critical parameters of stability, sensitivity, ease of use, and robustness needed are being addressed so that it is finding its place; for example, CE-MS has changed the way our laboratory approaches protein analysis, because of the speed, low sample requirements, sensitivity, minimal carryover, and robustness. But commercial development is critical, as most users are not going to develop their own systems.
CW: CE will continue to evolve because there is a constant and sincere drive to meet unmet analytical needs especially in R&D and production of new biopharmaceutical drugs. This is where CE-MS will have a lot of impact.
TS: In Japan, CE-MS based metabolomics is used widely not only in universities but in various types of industries. The greatest advantage of CE-MS is high quantification accuracy and it is hardly affected by matrix effects, such as ion suppression and enhancement, unlike LC-MS. In fact, if CE-MS sensitivity is improved, it might replace LC-MS!
RH: In some cases, CE-MS is already used routinely and it often provides faster separations than other methods. In protein characterization (this includes intact protein analysis, peptide mapping and glycan profiling, for example), I think it will become a more routine method, adding more selectivity to the analytical toolbox.
ND: This is an interesting question. I suspect that CZE will find a role in routine characterization of recombinant therapeutics within the next five years.
CW: The basis for CE method development is a good understanding of CE separation fundamentals as well as knowledge of substance properties and previous experience – and this lack of knowledge has presented obstacles. Today’s students are not trained extensively in separation science (both for HPLC and electrophoresis), which hampers the knowledge transfer from academia to industry. In addition, more officially approved CE methods (United States Pharmacopeia, European Pharmacopoeia, US Food and Drug Administration, etc.,) would be very helpful for a wider distribution of this technology.
ND: There are three issues. First, the review community at the US National Institutes of Health (NIH) is loath to support research on CZE-MS. Until the community supports investment in the field, progress will be slow. Second, the instrumentation industry is conservative in its investment in the field. Third (and related to the first), the pitiful investment in academic CZE research is leading to a very small base of trained researchers. As a result, the pharmaceutical industry finds it difficult to recruit scientists to develop high-throughput applications.
MK: Commercialized advances, awareness of CE advantages, robust CE capillary coatings, and reliability have hindered its acceptance. Current sample volume requirements on commercial instruments must improve for sensitive analysis too. If the disparity in vial volume to loading amount onto the CE is resolved, together with sample preparation and handling of small volumes, it should lead to a substantial improvement in sensitivity for CE-MS. Sample preparation methods also need to be developed in parallel to instrument development.
RH: For a long time, the lack of reproducibility has been an issue that has stigmatized CE-MS. However, I think that many recent studies prove reproducibility is not an issue. In addition, the lack of good commercial solutions for interfacing CE and MS has also contributed to the negativity, but this is also changing.
TS: Sensitivity, stability in analysis and correction software for migration time shift have all contributed to slow uptake.
RH: I am aware of two commercial approaches for CE-MS interfacing (see Figure 5). The Agilent interface has been on the market for about 20 years now and definitely is considered a robust way of interfacing. Unfortunately, it is only compatible with Agilent (Santa Clara, California, USA) and Bruker (Bremen, Germany) MS systems. Beckman Coulter (Brea, California, USA [now Sciex Separations]) recently introduced a new interface that is compatible with more MS brands. In addition, it shows improved sensitivity compared with others, but as it is new it needs to build a track record – and I am confident that it will. In addition, it is nice to see that academia is showing an increased interest in interfacing. For example, Norm and Dick Smith are both developing improved CE-MS interfaces and showing their applicability. Let’s hope we see more commercial solutions in the future. In my opinion, every MS vendor should have a suitable CE-MS solution – it would make the market more competitive and the technology would benefit.
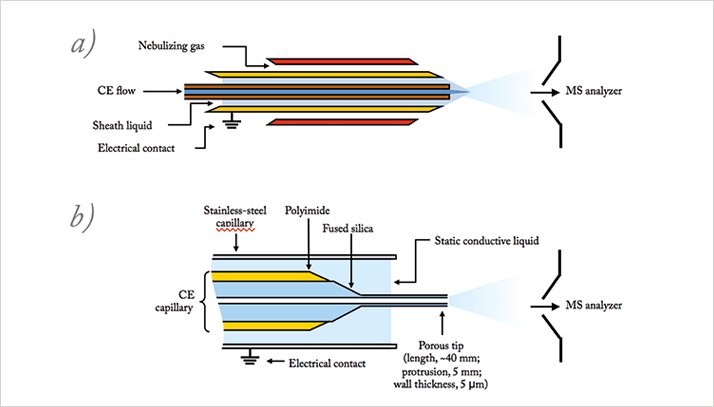
MK: The sheathless capillary electrophoresis-electrospray ionization (CESI) interface is a promising solution for high sensitivity analysis but it is integral to the capillary. Also, the triple tube interface is stable, easy to setup, accepts any capillary, but is not as sensitive as other interfaces. However, the nanospray electroosmotic flow (EOF) driven sheath interface appears robust, sensitive and accepts any capillary.
ND: My group has developed a very sensitive and robust CZE-nanospray interface and licensed it to CMP Scientific (New York, USA), a small startup that collaborates with Prince Technologies (Emmen, The Netherlands) and others for distribution. The interface appears to be successful in a number of applications.
TS: Most vendors – except for Agilent and Bruker – do not earth (ground) their CE-MS probes, which can cause extrusion of injected samples from the inlet capillary in both cationic and anionic modes. Today, a CE-MS sheath flow interface is commonly used, which enables very stable CE-MS analysis, but with decreased sensitivity. It’s also a complicated system (you need both a sheath liquid and a pump). I think that the next generation of this technology will be sheathless CE-MS, which may much improve sensitivity.
CW: Today, commercially available sheath liquid interfaces for CE-MS are robust, versatile and have sub-ppm detection limits. Standard dimension capillaries (off the roll) can be used with this technology. Commercially available sheathless interfaces, on the other hand, provide high sensitivity but these need non-standard, dedicated, and very expensive capillaries. We still need a CE-MS interface that combines high sensitivity with versatility and robustness at reasonable cost.
MK: To drive it forward over the next five years, we need more people working with CE-MS. It already has its place in intact proteins, protein complexes, and complex protein separations; the separation of intact proteins has driven me to look into the technique. I think that the issues of stability, sensitivity, ease of use, and robustness are being overcome, so they are much less of a problem and obstacle for the technique. The speed and low sample consumption of CE-MS analysis will increase uptake and I think cycle times of five minutes between injections are achievable. But in five years, don’t expect that CE peaks will be identified just by migration time.
ND: As the famous “philosopher” Yogi Berra once said, “It is tough to make predictions, especially about the future.” If investments are available, then CZE will find increasing application in industry. It is reasonable to expect CZE to find routine use in characterization of recombinant therapeutics, and to find growing acceptance in both top-down and bottom-up proteomic analysis.
TS: Let’s see more availability and use of sheathless CE-MS to improve sensitivity – and we need to develop (and make available) very stable and easy-to-use methods.
CW: I’m convinced that in the near future CE-MS will play a much more prominent role in many application areas. However, this technology will have its greatest impact on the characterization of biopharmaceutical drugs – an area where already today CE has a strong position.
RH: Good question! The current momentum is definitely in favor of CE-MS. So, the analytical chemistry community should keep it going. Obviously, we shouldn’t put unrealistic expectations on the technique as then it may result in disappointment (again). It has proven, and will continue to prove, very useful for certain applications and it should be used accordingly. If we do that, I think CE-MS will finally become the routine technique I already know it can be.