Performing highly sensitive, microscale LC-MS separations can be a complex and challenging task. Could we develop a microflow LC-MS system that not only provided high sensitivity, but was easy to use, robust and reproducible?
I work with customers in all types of labs across diverse organizations, whether it is a core facility at a major pharmaceutical company or a QC lab at a food processing company. I collect information, distill it, analyze the market potential, and deliver it to the organization so we can produce products that have a meaningful impact on our customers. When speaking with LC-MS customers at Waters Corporation, one challenge that frequently comes up is how to improve analytical sensitivity. Scientists today increasingly have a need to achieve lower limits of quantitation that can be driven by a variety of factors; for example, increasing regulations, the need to dose therapeutics at lower levels, the desire to find biomarkers at ultralow concentrations or the low volume of sample available. But even with ongoing advances in laboratory instrumentation, sensitivity demands may be greater than what can currently be achieved on a routine basis.
Many scientists have attempted to improve LC-MS sensitivity by reducing flow rates. It has been widely demonstrated that reduced flow rates combined with a smaller column diameter can achieve higher sensitivity when injecting the same amount of material. In addition to sensitivity gains, working with smaller size samples during microscale separations has additional benefits: reduced solvent and standards costs as well as solvent storage and disposal costs. However, in a production environment, microscale LC-MS can be extremely challenging to perform – if not impossible – despite the many benefits. In some ways, it’s an art rather than a science – a user must become efficient at making good connections and minimizing dispersion. Troubleshooting can be challenging as leaks are difficult to detect. And transferring a method from one instrument to another is not easy. Some of our customers tell us that it often takes weeks or even months to become proficient in setting up and operating a microscale LC-MS that meets their expectations.
One of my R&D colleagues at Waters, Jim Murphy (principle research chemist), told me a story that sums up the issue. He once walked into a lab of a major pharmaceutical firm and met a scientist who was struggling with low flow chromatography. “His set-up looked like a series of tinker toys,” Jim told me. “He had a column from one vendor, a column heater from another vendor floating out in space, an electrospray interface in front of a mass spectrometry system and he was clearly struggling. And yet, despite all of his efforts, he wasn’t getting the sensitivity he wanted.” It was a familiar story. And so, approximately seven years ago, we embarked upon a new project to change the game. Geoff Gerhardt, senior director of core research, sums up our initiative nicely: “We thought if we could realize the performance you get at the microscale with the same ease of use, or better than what you are used to at the analytical scale, that would present a huge opportunity for our customers. We wanted to maintain the same level of UPLC performance that we had just introduced – but in a format that could take advantage of microscale.” Here’s how we did it.
Increased sensitivity – and therefore microscale separations – were clearly in demand – but how could we make it more robust? We actually launched the first iteration of an integrated microfluidic separations device named TRIZAIC back in 2008. But the instrument was aimed mainly at proteomics researchers, who were working with 75 µm nanoscale chromatography and proficient at operating nano and microscale LC-MS systems. In 2010, we embarked upon project “Tesla” with three aims: to deliver high performance mass spectrometry, to utilize green technology to greatly reduce solvent usage, and to make the system easy to use. Indeed, the latter consideration was important right from the start, we were determined to produce a system that would offer microscale high sensitivity LC-MS with the same robustness and reproducibility that customers working with 2.1 mm ID (internal diameter) chromatography were used to.
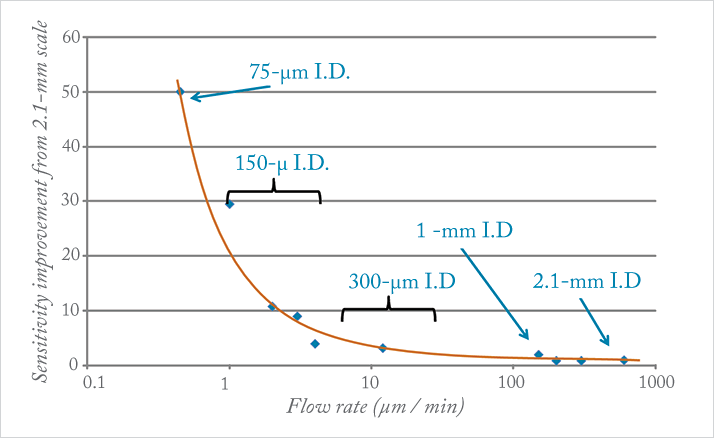
An integral part of the R & D team, I remember Jim Murphy saying, “We are not going to release this technology until we nail two things. First, it has to be easy for everyone in the lab to use – from LC-MS experts to technicians. Second, it has to be really robust.” In other words, we were not going to commercialize it until our key customers said it was ready. We began collaborating with scientists who worked in the world’s most demanding laboratories in drug discovery and development, contract services, food and environment, academic research as well as core labs. We experimented with various chromatographic channel IDs. We learned that although 300 µm channels provided higher sensitivity than 2.1 mm columns, it was typically not enough of an improvement to capture the attention of customers. Further experimentation showed that 150 µm channels provided sufficient sensitivity but still offered similar throughput and robustness of 2.1 mm columns (see Figure 2).
One of our collaborators on the project, Phil Tiller at RMI Laboratories, who tried the 150 µm said that he was blown away by the increase in sensitivity. “Three of our DMPK scientists got more than an eight-fold improvement in sensitivity compared to 2.1 mm ID chromatography on our first attempt.” We had found the “sweet spot” for integrating LC-MS. Our R&D team worked closely with Phil and other scientists – exchanging ideas, running samples side by side in our labs, and providing the technology to collaborators for them to use in their laboratories. Such collaborations were invaluable to the project. One of the greatest benefits of working in collaboration with our customers during the project was that they challenged the system in ways we never expected and tested the system for a wide range of applications we had never even considered. And that opened our eyes to the broad applicability of the system. It also helped us learn and focus on where product improvements were needed. Our collaborators took a chance to work with us and were critical to the development process.
In 2012, we held an R&D summit, bringing all of our collaborators together in one location. The enthusiasm was infectious and we knew that we had something that could change the way people performed LC-MS. In the ensuing years, we dedicated a team to do everything necessary to release the product. We secured our supply chain for critical raw materials; we challenged the system with tens of thousands of injections; and we tested various systems with a number of users looking at robustness and reproducibility intra and inter system. We commercially launched the ionKey/MS system at Pittcon 2014, coupling it with the Waters ACQUITY UPLC M-Class and the Xevo-TQ-S mass spectrometer, making the most sensitive MS accessible to everyone in the lab. Since the commercial launch, we have expanded ionKey/MS to the Waters Synapt G2-Si and the Xevo G2-XS QTof mass spectrometers. Ultimately, what we brought to the market was a fully integrated LC-MS system that provides reproducible and robust UPLC separations, with up to 40 times greater sensitivity than standard columns – day in and day out. And its truly plug-and-play nature means virtually anyone can use it.


Indeed, perhaps the most innovative aspect of ionKey/MS is the iKey (see Figure 1), which replaces the column, column heater, electrospray emitter, and simplifies the user experience tremendously. The iKey is about the size of a smart phone and incorporates a rigid monolithic substrate made of ceramic, chosen for its strength and inertness. The ceramic substrate is inscribed with a 150 µm channel packed with 1.7 µm UPLC chromatographic particles. The ceramic substrate is then encased in a housing that contains the column heater, all electronic connections and an electrospray emitter. When the iKey is locked (see Figure 3) into position at the source, all of the electronic and fluidic connections are made automatically, thus eliminating any potential variabilities. The sample is then introduced to and separated in the iKey and transported directly to the integrated emitter, which converts the eluent into an aerosol. The plume of fine droplets in the aerosol are ionized giving them a positive charge at which point they enter the vacuum of the MS where they are further separated.
Ceramics are commonly used in the computer industry but have never been intimately coupled with LC-MS. So we had to rely on partners for R&D and manufacturing. Because we integrated so many components into the iKey and the source of the mass spectrometer, managing the number of variables was extremely challenging. When we wanted to test the effects of different variables during the development process, we often experienced very long turn-around times to get new modified devices to test our assumptions. Over time, Waters made a significant investment into people and equipment to better understand the ceramic manufacturing process. Now, virtually all aspects of the iKey manufacturing are performed in a carefully controlled facility at Waters headquarters in Milford, Massachusetts. In doing so, we’ve developed a manufacturing center of excellence around micromachining to tight tolerances, created a unique ceramic processing technique, and developed an innovative column frit technology. Bringing the technology in house, we felt, was crucial to later success.
Today, after nearly one year on the market, ionKey/MS customers are telling us that they are collecting data that they couldn’t hope to get before – but also that the system is easier to use and more robust than they ever expected. One customer at a leading pharmaceutical company told us, “This is not nice-to-have technology. This is must-have technology”. Praise indeed. And it means that we’ve hit the mark: this technology could help researchers advance research and help get therapeutics and treatments to market faster. Personally, I’ve learned several things from this ambitious project. Product and technology development is a marathon rather than a sprint – and the path to product commercialization is one of fits and starts. The whole process of innovation is one of mutual dependency: scientists depend on instrument vendors to advance the technology that will help them in their research while instrument vendors like Waters depend upon scientific collaborators to validate concepts and reduce the risks inherent in commercializing products. Without each other, we would undoubtedly see fewer innovative products.
Peter Claise is product manager for the ionKey/MS System at Waters Corporation, Milford, Massachusetts, USA.
