
Nadia Elghobashi-Meinhardt

Svea Stephan
The COVID-19 pandemic underscored the need for effective vehicles to deliver genetic material – such as mRNA – into cells. As a result, lipid nanoparticles (LNPs) rose in prominence and were used for several mRNA vaccines for COVID-19. This demand, which is also being driven by cell and gene therapy developers, has led to the rise of alternative encapsulation and delivery vehicles, such as the small-volume particles produced by co-precipitation-crosslinking-dissolution (CCD).
KNAUER is collaborating with the Charité research hospital to automate CCD production using their benchtop NanoScaler solution. They’ve recently published an application note describing a method by which protein metal carbonate particles (0.1-10 micrometer scale) can be precipitated using CCD – and the NanoScaler.
Here, we speak with Nadia Elghobashi-Meinhardt, Project Manager, and Svea Stephan, Application Scientist – both at Knauer’s Customized Solutions division – to find out more about the collaboration.

Could you give me an introduction to the NanoScaler?
Elghobashi-Meinhardt: The NanoScaler is a benchtop instrument that KNAUER introduced in 2022 for lipid nanoparticle research and development to screen for optimal process parameters. Its main features are three pumps and an impingement jets mixer (IJM) mixing chamber. The instrument can be used for a wide range of applications, but the main application to date has been the production of lipid nanoparticles (LNPs), in which the payload or active pharmaceutical ingredient (API) is a nucleic acid, such as RNA or DNA. The pumps deliver the components (API, lipid) to the IJM, where these mix to form LNPs. A third pump can be used to dilute the LNP solution.
Stephan: The NanoScaler’s IJM technology allows researchers to determine optimum encapsulation conditions before they scale up the process using the same mixing technology in KNAUER’s production systems. In addition to LNPs, the NanoScaler can also be used to produce liposomes, nanoemulsions, and microparticles.
What is co-precipitation-crosslinking-dissolution?
Stephan: CCD is a technique that allows the fabrication of particles composed of different biopolymers and bioactive agents – not just lipids – under mild conditions without the need for any organic solvents. It is a multi-step process that begins with mixing inorganic salt solutions, such as MnCl2 and Na2CO3, which results in the formation of water-insoluble metal carbonate template particles that encapsulate biopolymers. These hybrid particles are then crosslinked to stabilize the biopolymers within. Subsequently, the inorganic template is dissolved, leaving behind pure biopolymer particles.
Why is the NanoScaler a good fit for small-volume particle production?
Elghobashi-Meinhardt: The NanoScaler is a good fit for R&D and small-volume particle production because its benchtop format is easy to incorporate in a lab and the software allows for easy method development. In general, the NanoScaler is a good option if two components are being mixed, so beyond LNP production, other applications, such as the CCD technique, are feasible. Since the COVID-19 pandemic, the number of LNP formulation studies – for vaccine development but also for new drug candidates – has increased exponentially.
Stephan: The setup of the NanoScaler is optimized for low sample consumption, to avoid wasting valuable API or lipid. Both substances are very cost-intensive and, especially at the beginning of a method development, developers want to work with the smallest possible volumes. Additionally, the NanoScaler is very flexible regarding the mixing unit. It is possible to switch within one minute to a different IJM or even connect other mixing technologies.
Tell me about the collaboration with Charité – how did this come about?
Elghobashi-Meinhardt: The Charité is a world-renowned research hospital that is, like KNAUER, located in Berlin. The collaboration came about because the research group at the Charité wanted to automate their CCD technique and asked us if we would be interested in joining forces. KNAUER is always strongly interested in supporting innovative research.
Stephan: There is a need for a standardized process for co-precipitation that complies with "good manufacturing practice" (GMP) and that is scalable and automatable – we felt that the NanoScaler would be a good fit here.
I’d just add that the research department of the Institute of Transfusion Medicine at Charité-Universitätsmedizin Berlin has many years of experience and expertise in the fields of biophysics, cell biology, biotechnology, and physical chemistry. The current research focus of the working group is on the development of drug delivery systems, the development of blood substitutes and the investigation of the immunological properties of micro- and nanoparticles and the interaction of these particles with blood cells. We’re excited to be working with them!
Where are you up to with the collaboration?
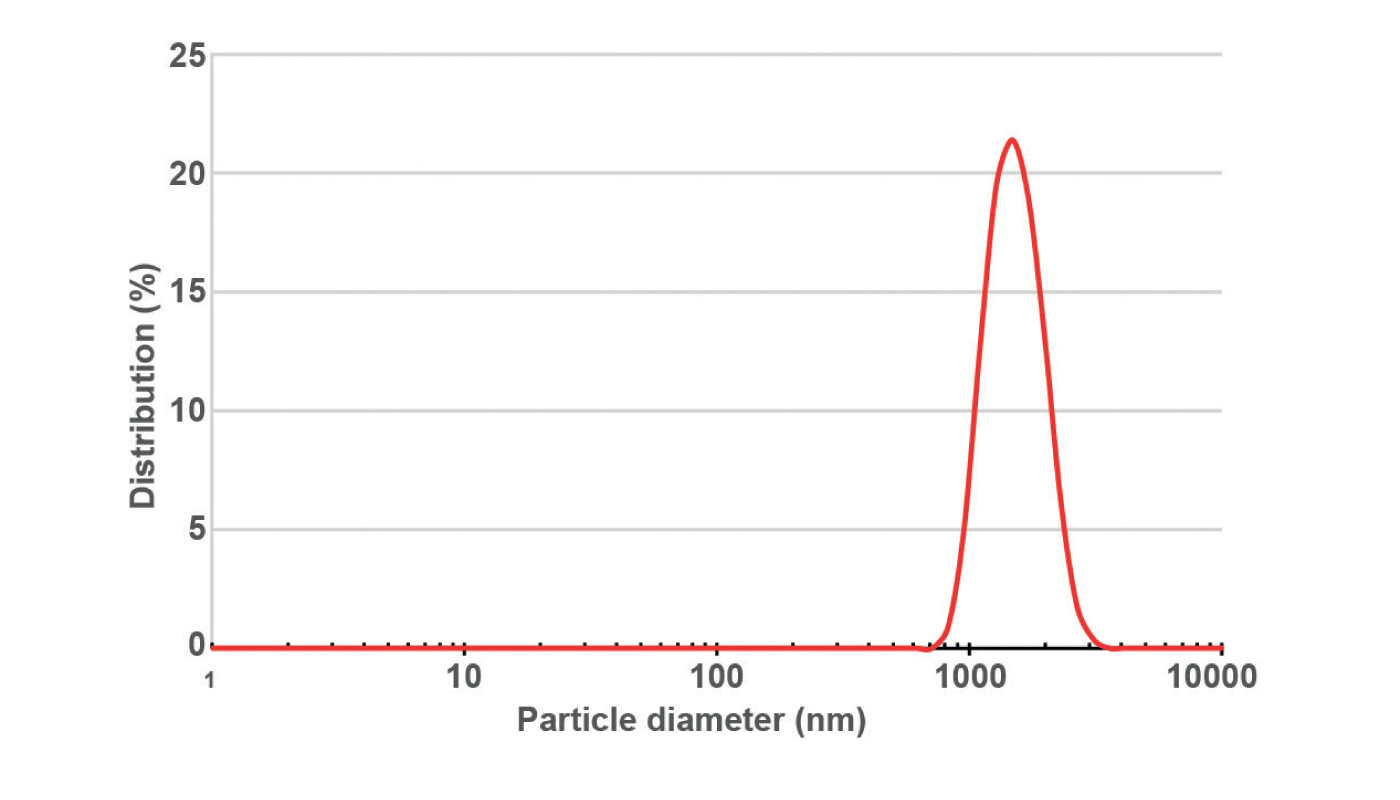
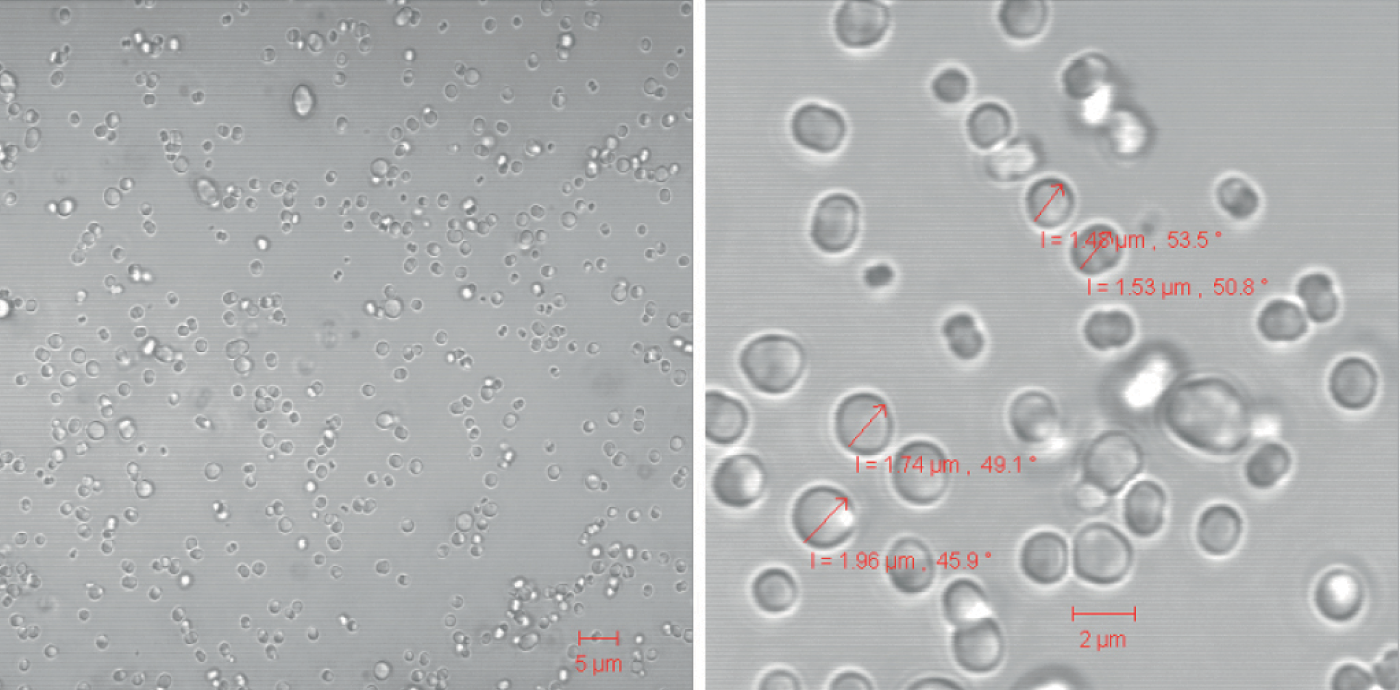
Elghobashi-Meinhardt: The collaboration is still active! Currently, the NanoScaler produces metal carbonate templated protein particles, but we are optimizing the parameters to modulate the size of the particles which currently have a diameter on the order of several micrometers. We are curious if we can change the particle size by changing flow rates and concentrations.
Stephan: We’ve published an initial app note with Charité where we investigated whether the traditional co-precipitation step of the CCD method, in which bulk solutions are manually mixed in a beaker, can be replaced by automatized mixing with the NanoScaler’s IJM technology. We were able to show that the KNAUER IJM NanoScaler can be used to formulate protein metal carbonate hybrid particles by mixing two inorganic salt solutions (MnCl2 and Na2CO3) in which the protein is also dissolved. Suitable additives (for example, Dextran 70) allow the production of these hybrid particles in µm range as single particles with only a small number of aggregates. In contrast to results obtained from manual production, the results from the NanoScaler are reproducible.
You can download the app note here.
Do you have plans for future experiments in this area using the NanoScaler?
Elghobashi-Meinhardt: Yes, we have ongoing experiments and are actively planning future experiments that include adjusting the geometry of the IJM to optimize the mixing process.
Stephan: The flow conditions required for the precipitation process must be controlled and regulated, which will also be addressed in a sub-project. As the project progresses, an IJM system that continuously produces biopolymer particles will be developed and tested. A scale-up of the process is also planned.





