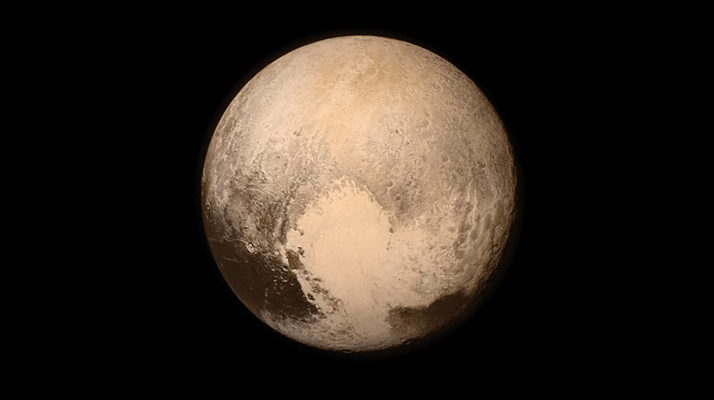
The New Horizons spacecraft has just successfully conducted its first flyby of Pluto – and there are jubilant scenes in Mission Control as we write this on July 14, 2015. After a nine-year journey of more than three billion miles, expectations are certainly high – and, so far, those expectations are being spectacularly met. Surprise number one: Pluto is bigger than we thought. Surprise number two (breaking news): Pluto is red, just like Mars. But the flyby itself shouldn’t take all the credit. To help the New Horizons science team interpret the incoming spectra, analytical scientists from Northern Arizona University’s (NAU) ice lab have been growing and analyzing ice samples that simulate Pluto’s surface. The group hopes to understand how composition, temperature, and ice phase affect the absorption coefficient spectra of CH4 and N2 ice mixtures – the dominant species on the surface of Pluto. Stephen Tegler, professor and chair of NAU’s Physics and Astronomy Department and overseer of the ice lab, tells us more about the work.
How did you get involved with the ice lab and NASA? My first professional contact with NASA was as a graduate student. My PhD thesis concerned measuring the abundance of NH3 in comet Halley. Almost ten years ago, I began a program to obtain optical spectra of icy dwarf planets using the 6.5-meter MMT telescope on Mount Hopkins in Arizona. I needed laboratory data of ices to interpret the telescope data, so I began collaborating with two colleagues who were building the ice lab, William Grundy of Lowell Observatory and David Cornelison, then of NAU. When Cornelison left NAU, I began to take a bigger role in the ice lab. Since Grundy is on the New Horizons spacecraft team, we began a campaign in the lab to better understand CH4 and N2 ice. One purpose of the ice lab from the start was to maximize the scientific return of icy dwarf planet spectra taken with ground-based telescopes, as well as Pluto spectra returned by the New Horizons spacecraft.
How do you fingerprint the ice samples? We collect optical and near-infrared spectra of ice samples with a Fourier transform infrared spectrometer (FTIR) and use the MATLAB programming language to remove instrumental and atmospheric signatures in the data. Our final products from the lab data are absorption coefficient spectra. We determine the effect of composition, temperature, and ice phase on the absorption coefficient spectra. How difficult is it to translate findings here on Earth to an alien planet? Our program requires the collection of astronomical spectra from a telescope or a spacecraft. The astronomical spectra must be processed to leave only the signature of the astronomical source. Laboratory data must be collected and processed as well to yield absorption coefficient spectra. Finally, we use a radiation transfer model, the Hapke model, to relate the laboratory absorption coefficient spectra to the astronomical spectra. The Hapke model takes into account the scattering of light in the surface of the icy dwarf. In summary, our program requires laboratory, telescope observing, radiation transfer modeling, and computer programming skills. So to answer the question – it’s very difficult!
What have been the most surprising findings so far? We were able to measure the CH4 and N2 abundances on Pluto and Eris using telescope observations and laboratory experiments and found the two bodies have similar CH4 and N2 abundances. Are you excited about New Horizon’s flyby of Pluto in July? Yes, I can’t wait to see the high-resolution images of Pluto and Charon’s surface! The solar radiation field should destroy CH4 ice on the surface of Pluto. So, there must be a source replenishing the CH4. I hope New Horizons will help us understand the replenishing source.




