A research group from the University of Pennsylvania have developed a portable, low-cost and easy-to-use test that could help with diagnosis of the Zika virus (ZIKV). The Micro and Nano Fluidics laboratory (http://bau.seas.upenn.edu/) has made significant advances in point-of-care (POC) diagnostics, but it was the recent outbreak of ZIKV in the Americas that kickstarted the current research.
“When we learned about the virus and its devastating consequences for pregnant women and their fetuses, as well as the lack of appropriate diagnostic tools, we recognized that we were in a position to provide an appropriate solution,” says Changchun Liu, Research Assistant Professor (Department of Mechanical Engineering and Applied Mechanics at the University of Pennsylvania’s School of Engineering and Applied Science). RT-PCR (reverse transcription polymerase chain reaction) assays – the only laboratory-based molecular tests approved by the Centers for Disease Control and Prevention (CDC) for use on an emergency basis – require a well-trained technician and expensive instrumentation, conditions that are not optimal for POC diagnostic applications. Therefore, there is a strong need for a new diagnostic technique. “Generally, lateral flow tests suffer from low sensitivity. They also suffer from low specificity, since antibodies to ZIKV cross-react with other flaviviruses prevalent in Zika-endemic areas,” says Liu. “For these reasons, lateral flow tests are not recommended by the CDC and, as far as we know, are not used in South and Central America for diagnostic purposes.” Liu says that reverse transcription-loop mediated isothermal amplification (RT-LAMP) could be a highly sensitive (detecting the presence of the ZIKV before antibodies appear) and specific alternative to RT-PCR. “We developed a simple, sensitive RT-LAMP assay. RT-LAMP is also more suitable for POC applications because it is isothermal, can be carried out with minimal or no instruments, consumes less energy, and is more inhibitor-tolerant than PCR.” The group chiefly focused on two distinct facets: i) the design of new RT-LAMP primers for ZIKV detection, and ii) the implementation of the assay in a microfluidic format suitable for POC applications with minimal instrumentation. Using data mining, they identified highly conserved regions of the ZIKV genome that are divergent from other known pathogens. They then designed appropriate primers to recognize this sequence. The result? An assay suitable for applications in the laboratory and in the field, as well as a low-cost POC system that consists of a diagnostic cassette and a processor composed of a chemically-heated cup (see Figure 1) that can be operated by minimally trained personnel – without the need for electricity.
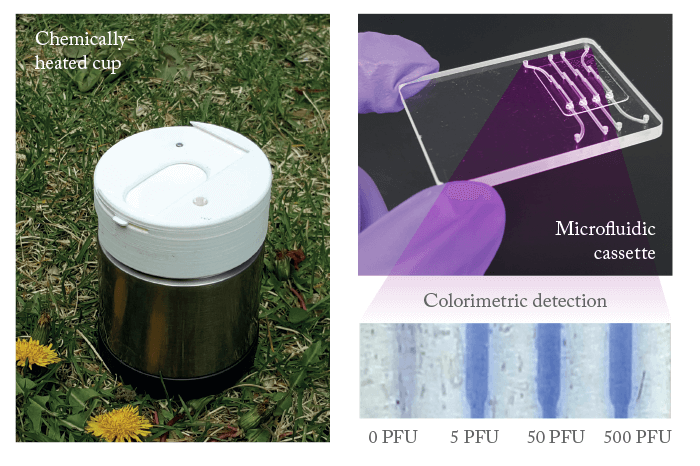
“The cassette isolates, concentrates and purifies nucleic acids, and carries out enzymatic amplification,” explains Liu. “The results are quantified with a leucocrystal violet (LCV) dye that changes from colorless to violet in the presence of double-stranded DNA.” The processor is also able to interface with a smartphone for signal excitation, acquisition, processing, archiving, and transmission (including GPS coordinates and a time stamp). To test the assay’s efficacy, the researchers collected saliva samples from healthy adult volunteers and spiked them with known amounts of ZIKV. After inactivation, the virus was filtered through an isolation membrane. The RNAs bound to the membrane then served as templates in the RT-LAMP amplification process without the need for an elution step, reducing the number of operations and simplifying flow control. The flow-through design of the reactor decoupled the sample volume from the reaction volume, enabling the group to use relatively high sample volumes and achieve high sensitivity. At the moment, the work is at the “proof of concept” stage and needs to be tested more extensively before the assay can be adapted for medical use. Says Liu, “We must experiment with patients’ samples and ensure that our assay and system match the performance of the gold standard (laboratory-based RT-PCR) and operate reproducibly and reliably.”
References
- J Song et al., “Instrument-free point-of-care molecular detection of Zika virus”, Anal Chem, [Epub ahead of print] (2016). DOI: 10.1021/acs.analchem.6b01632.




