The teamwork of surgeons and analytical scientists became a hot topic in our February issue (tas.txp.to/0215/precisionmed). From the efforts of the Maastricht MultiModal Molecular Imaging Institute to technologies, such as the iKnife and Verisante’s ‘parking sensor’ technology, it seems that the race is on to bring analytically-enhanced tools into clinics and operating rooms, particularly those tools that can differentiate between cancerous and normal tissue. Now, two more research groups have published work describing Raman-based technologies and how they can potentially aid cancer removal.
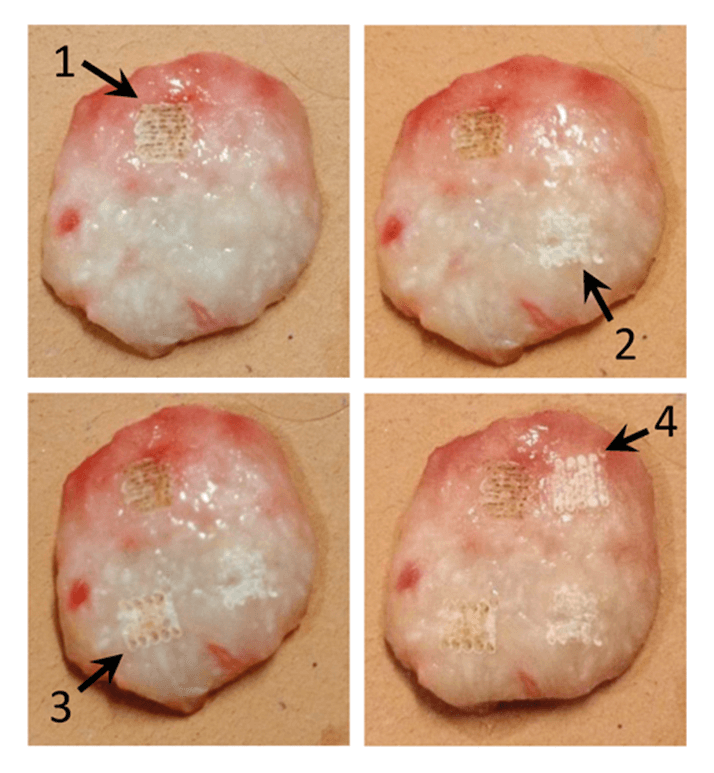
At Florida Atlantic University, a team of researchers has focused on non-melanoma skin cancer (1). The treatment of choice at the moment is Mohs micrographic surgery (a specialized excision), but the researchers argue that this is not always necessary or feasible. Their suggestion is to combine C02 laser ablation (see Figure 1) with Raman spectroscopy. Laser ablation offers precise tissue removal, with the potential for less scarring and almost bloodless surgery, but it is difficult to confirm if all the cancerous tissue has been removed. Raman spectroscopy, however, could be used in situ following partial laser ablation. The study has been a success, with the team developing a spectral classification model based on principal component analysis and binary logistics regression that could correctly identify squamous cell carcinoma tissue with 95 percent sensitivity and 100 percent specificity following partial laser ablation. The group hopes the work will clear the way to bringing guided laser-ablative procedures into the clinic. Andrew Terentis, lead scientist on the study, tells us more.
How did you get involved in this work? I have to credit Hugh Beckman (co-author on our recent paper) for the original idea of combining laser ablation with Raman spectroscopic diagnosis. Hugh is an ophthalmologist who has worked with various forms of laser treatments over the years. He’d heard about Raman spectroscopy and wondered whether it could be useful when combined with laser ablation. He searched for a Raman spectroscopy expert that could test the feasibility of this idea and eventually came into contact with me. Since my group was already working on the use of Raman spectroscopy to diagnose skin cancers, it was natural for us to pursue this project. John Strasswimmer is the other crucial partner in this research since he is the clinician that provides the patient access and skin cancer specimens we need for study. Any outstanding work from other groups? linician that provides the patient access and skin cancer specimens we need for study. We are familiar with the excellent work being conducted by the groups in Canada, which you covered in February (tas.txp.to/0415/surgical). I also believe that Gerwin Puppels’ group in the Netherlands has done transformative work in the area of adapting Raman spectroscopy for use in surgery. In the UK, Ioan Notingher’s group has also done some nice work with using Raman spectroscopy to diagnose skin cancers. There are other groups as well, but these are the ones that stand out for me at present.
What are the main challenges? The real challenge is yet to come: developing the technology into something clinically useful for the future. Raman spectroscopy is useful because it provides a lot of structural information – a “fingerprint” pattern of the biochemical content of the tissue. However, normal Raman spectroscopy is a comparatively low sensitivity technique and it does take a long time to scan a large area of tissue. If this is to be implemented clinically in the future, the speed of real-time Raman data acquisition and computer processing of the data needs to be improved and optimized. Thus, there are a multitude of engineering as well as computational challenges involved. What does the future hold? We are currently consulting with possible industry partners that can work with us to develop a clinical prototype. And although more technologically challenging, the treatment of any type of internal cancer using robotics to guide the ablative/cutting laser and a Raman probe to differentiate normal from cancerous tissue could be feasible in the future.
Meanwhile, researchers at the Montreal Neurologic al Institute and Hospital, McGill University and Polytechnique Montréal, have focused their work on an intraoperative probe for use during brain surgery designed to detect cancer cells at cellular resolution and inform surgeons whether the removal of cancerous tissue is complete (2). The probe relies on Raman spectroscopy and is about to enter a clinical trial in Montreal. One of the study’s senior authors, Kevin Petrecca from the Brain Tumour Research Centre at Montreal Neurological Institute and Hospital, is already using the probe routinely. Frederic Leblond, co-senior author and Professor in Engineering Physics at Polytechnique Montréal, answers our questions.
What is fueling recent interest? The development of Raman spectroscopy as a diagnostic tool for cancers and other tissue abnormalities has held great promise for decades, but the field has been prevented from reaching its full clinical potential by significant technical challenges. The Raman effect that allows us to use optical properties of tissue to distinguish pathologies is very subtle, requiring very sensitive instrumentation, long acquisition periods and advanced classification algorithms. Until now, the use of Raman spectroscopy has been limited to pre-screening diagnostics, pathology samples and animal models.
How did you get started? Grade 2-4 gliomas are inherently invasive cancers. As the decreasing gradient of cancer cells invades the brain, it is not possible to distinguish a boundary between cancer and normal brain, leading to local recurrence in around 85 percent of cases. Our group has extensive prior experience in the development of image-guided neurosurgical techniques, including magnetic resonance imaging and fluorescence, and the limitations of existing technology prompted us to consider newer, more specific and sensitive molecular detection techniques. This led us to investigate the integration of Raman spectroscopy into the neurosurgical workflow. Cancer is a very heterogenous disease and specific and sensitive tissue classification requires techniques that are able to highlight several molecular processes simultaneously. Raman spectroscopy potentially allows the identification and quantification of a very large number of molecular species and represented an excellent candidate for brain tumor detection. We have now already been able to demonstrate clinical proof-of-concept using the probe intraoperatively – in vivo - for over 30 patients with varying tumor grades and types. The probe is also easy to use not only because of its small footprint but also because inelastic scattering signal detection is in real-time taking a fraction of a second, i.e., 200 milliseconds. Moreover, the tissue volume that is interrogated by the probe is approximately 0.1 mm3, which is consistent with the volumes of tissue removed with the microsurgical dissection techniques used in neurosurgery.
Could the probe be used in other types of tumor surgery? Yes – we have data to demonstrate that this technology is not restricted to gliomas. It can be adapted to function in various clinical settings and can be used to identify different pathologies in real-time.
References
- S.A. Fox et al., “Raman Spectroscopy Differentiates Squamous Cell Carcinoma (SCC) from Normal Skin following treatment with a high-powered CO2 laser,” Lasers Surg. Med., 46, 757–772 (2014). M. Jermyn et al., “Intraoperative brain cancer detection with Raman spectroscopy in humans,” Science Translational Medicine, 7 (274) (2015).




