Analytical labs, especially in the pharmaceutical industry, are embracing accessible, community-suppor ted, interoperable data and communications standards such as AnIML and SiLA. AnIML is human readable, clearly structured, flexible, royalty free and available to all. SiLA enables communication between instruments and software systems to let you use your data however you want. Integration of these two standards is creating a new ecosystem – one that allows end-to-end integration of instrument control, data capture, and leading systems such as LIMS, enabling visualization of a sample’s entire lifecycle.
But let’s take a step back. One barrier to this vision of the lab of the future is that instrument data do not come in AnIML format out of the box (1). Much of the software available today was built years before the currentday appreciation for shared formats or standards, when companies developed their own formats. This has resulted in instrument data often being proprietary, closed and in non-readable file formats – the very antithesis of FAIR (2).
And this is exactly where data converters and connectors come in. First, a MilliporeSigma data converter reads and decodes the information from the proprietary format. This includes metadata such as instrument name, software version and experiment timestamps; and sample data, which are often just series of characters. How do we decode those data from the proprietary format? If they are human readable, we can fairly easily build a reader to convert them to AnIML’s XML format. If not, we work with vendors to solve the problem: they know what’s in their data, and they have software we can use to extract those data from their machines.
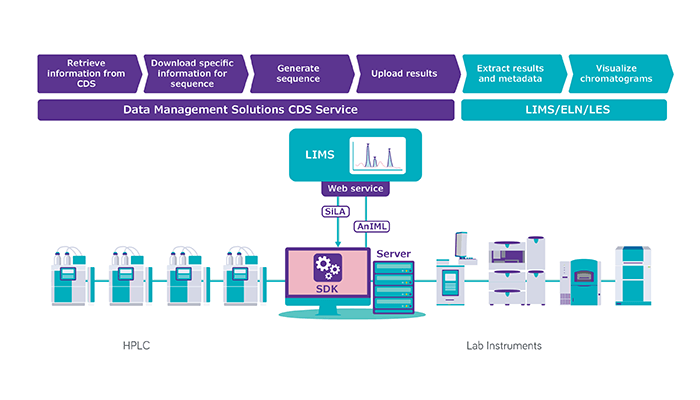
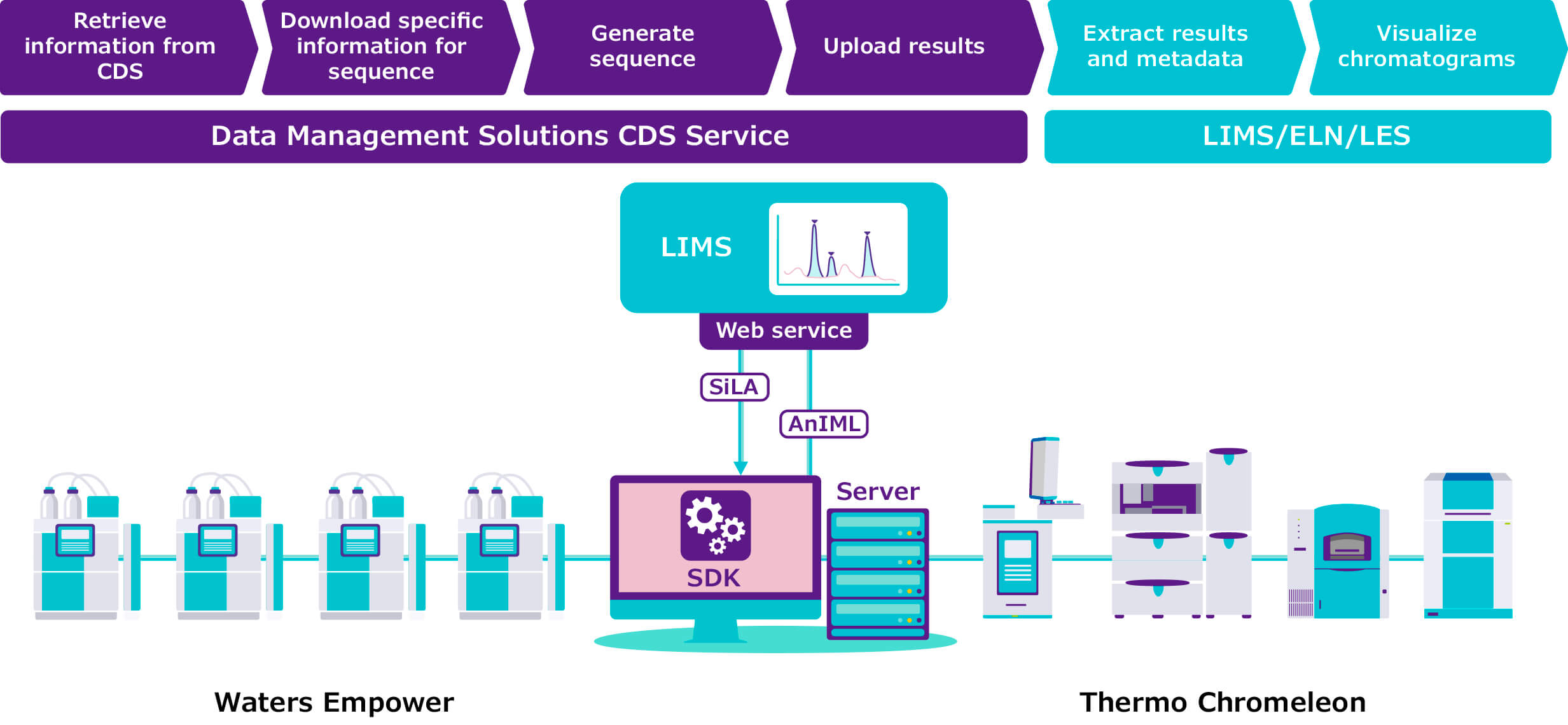
Next, the converter creates a semantically identical copy of the data in AnIML format and writes this copy to disk. This includes separate AnIML “containers” with sample data, metadata, audit trails and digital signatures. The result is an AnIML file you can use with any XML-compliant software tool.
Connectors are a little more complicated. Once your data have been converted into an AnIML file, you may want to connect that file to a software system such as LIMS or ELN. For this to happen, not only do you need to translate the instrument data to AnIML format, but you also need to teach the third-party software to read the AnIML file. Without disrupting the different software applications already used in the lab, our connectors work bidirectionally, creating interfaces between the AnIML files and the LIMS, ELN, or lab execution systems you have in place without changing how you use those systems.
The converters and connectors run in the background to automatically track and translate instrument data into AnIML files, which are then saved either locally or to the cloud. At this writing we have converters for more than 300 instruments, including for Thermo Fisher Scientific Chromeleon 7 and Waters Empower 3 (both of which support HPLC, GC, IC, CE and MS) and for Metrohm tiamo and its titration software. On the horizon are connectors for Agilent OpenLAB CDS 2 (anticipated Q4 2022), Metrohm OMNIS (replacing tiamo) and Mettler Toledo LabX.
The lab of the future – today
MilliporeSigma’s “Lab Automation” project illustrates how data converters and connectors can work in practice. MilliporeSigma’s Global Analytical Services group is an association of 21 labs across the globe that offer a wide variety of services in the healthcare and life science industries. The group supports more than 220 analytical methods with a wide spectrum of analytical equipment, such as HPLC, GCMS and NMR. The aim of Lab Automation was to create interoperable, bi-directional exchange of information between all heterogeneous systems and instruments to support analysts in their workflows (3) – the perfect use case for MilliporeSigma’s data converters and connectors. By translating all proprietary instrument data in Global Analytical Services labs to AnIML and collecting them in a central database, we achieved fully automated data flow, report generation and LIMS upload. In short, all data is captured electronically where it is generated, made available where it is needed and centrally stored using Data Management Solutions Hub software.
After one year, the Lab Automation project had successfully completed the digital transformation of three different Global Analytical Services lab facilities. What did it take? AnIML data converters for more than 20 different scientific instruments, bi-directional software integration with 4 different leading systems and 11 different measurement interfaces, a central data store (Data Management Solutions Hub), and a universal data analysis and visualization tool (Data Management Solutions Workbench).
The project team closely monitored the group’s output before and after digital transformation to quantify the project’s impact over the first year. Remarkably, Global Analytical Services saved a combined 3,386 working hours across all three labs, an estimated financial savings of approximately €400,000 and a nearly 20 percent increase in samples processed thanks to the automation of key workflows and data processes.
Terms such as “automation” and “digitalization” are buzzwords today, but the important concept underlying them is connecting things in a way that brings value to the customer. Converting locked instrument data to harmonized, standard formats like AnIML is the first step and a valid use case in its own right. But now, using our connectors, we can potentially take all instruments used in a lab, or in a group of labs across the world, and bi-directionally integrate them with a range of software systems to simplify and standardize forms, procedures and data flows in a way that also lets scientists visualize all the data in one system. These data can then be easily shared, reused and reanalyzed by different scientists, labs or companies.
That’s what Open Science is all about. The result, as our Lab Automation project shows, is greater efficiency, significant cost savings and more freedom for scientists to do what they do best: conducting experiments and interpreting the results.
Data converters can be especially useful for pharmaceutical companies in their interactions with regulatory agencies. Companies often have data associated with the efficacy of their drug product that regulators wish to examine closely – which is impossible if those data exist in an unreadable file format. Manually translating such files, sometimes hundreds of thousands of them, to a human-readable format is a task no one wants to do. Fortunately, we can build a converter that automatically converts these files to AnIML for easy storage and availability to regulators. Data integrity is another important factor to consider from a regulatory perspective. AnIML has a built-in audit trail that lets you see first-hand whether a data file was opened, by whom and what they did with it, all while ensuring the raw data remain untouched.
References
- Arne Kusserow, “Welcome to the Lab of the Future,” The Analytical Scientist (2022). Available at: https://bit.ly/3RNtS1q
- Frauke Leitner, “Let’s Make Data FAIR,” The Analytical Scientist (2022). Available at: https://bit.ly/3BMwN4A
- MilliporeSigma, “Enabling Analytical Lab Automation & Data Harmonization” (2021). Available at: https://bit.ly/3QNuqTB





