Existing atmospheric pressure ionization-mass spectrometry (API-MS) instruments are unable to analyze low-polarity compounds. How can the advances made in ionization sources for liquid chromatography (LC) be extended to gas chromatography (GC)?
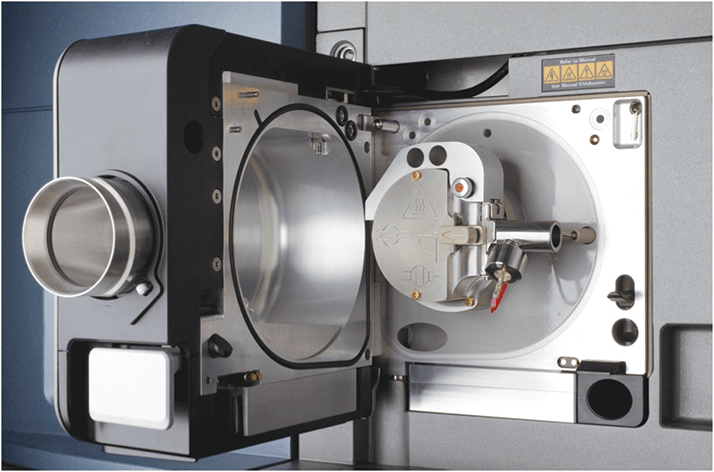
Mass spectrometry has increasingly become a key part of the modern analytical laboratory. But MS systems are a large investment for many organizations, so they must be used as efficiently as possible. One way to ensure optimal use is to maximize the number of compounds that can be analyzed per instrument by interfacing both LC and GC to a single MS platform. Let’s go back to 1973, when the technique of atmospheric pressure chemical ionization (APCI) was initially developed by Horning et al (1). APCI showed excellent promise for the ionization of relatively non-polar analytes, but lacked commercial instrumentation. Over time, ionization modes were developed for API-MS instruments, often combining multiple ionization techniques into one source design. Although this enabled a much wider range of analyte polarities, the technique was limited to compounds amenable to liquid chromatography. So, what about gas chromatography?
With that question in mind, many began seeking the elusive “universal detector”. Though there has been a general trend towards LC, many analyses simply work better with GC. Therefore, the option to use either separation technique with one MS product is a real bonus for a lot of laboratories.
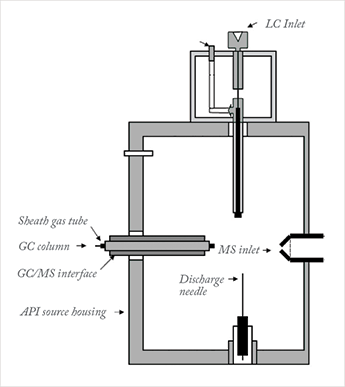
Fast forward to 2007, and Charles McEwen (then at DuPont) developed two new technologies: the atmospheric solids analysis probe (ASAP) and atmospheric pressure gas chromatography (APGC, see Figure 1). The ASAP probe allowed direct analysis of solids and liquids in an API source and APGC offered GC capability on an LC instrument (2). Charles (known as Chuck to his old friends) approached Waters to discuss these two new technologies, initially without much success.
Many new technologies are evaluated by Waters and we typically need to answer two fundamental questions: does it work in the real world (outside of R&D) and is there a commercial need? New technology always has a number of barriers to overcome and several innovations have come along, promised miracles, and (sometimes unsurprisingly) failed to deliver on those promises. However, good data tend to win people over, and so, about a year later, Michael Balogh (CoSMoS president and consulting principle scientist at Waters) heard about Chuck’s work via the CoSMoS (Conference on Small Molecule Science) committee. Michael convinced Andy Jarrell (a scientific fellow) to investigate APGC further. After some communication with the MS team in Manchester, UK, it was decided that the technology could be a good fit with Waters’ current and planned portfolio. Waters and DuPont agreed an exclusive license agreement and work commenced on both sides of the Atlantic in an attempt to turn the rough concept into a commercial reality.
The process of taking an idea from the prototype stage to a commercial product can be long and fraught with challenges. Our design philosophy – engineered simplicity – is to create products that are fit for the purpose intended but also easy for both customers and engineers to use and maintain. At the feasibility stage, various source geometries were trialed. The original source was open to atmosphere, but controlled ionization requires a closed source, which allows for a wider range of compound ionization possibilities (dry source for charge transfer and ‘wet’ source for proton transfer). The Universal (API) source was an existing closed source and deemed suitable for modification. After much trial (and some error) the geometry was settled, and in 2010 Waters filed for a patent of the modified design. The design uses a mini ionization chamber to provide a 30-fold sensitivity improvement on Chuck’s already impressive design.
An important aspect of the design was that it could be integrated into Waters’ portfolio of instruments. That is to say, the swap from LC to GC (and back again) needed to be as simple as possible. Past generations of instruments that could run with both LC and GC have required serious compromises (often in both modes). It was important to us that this system would be different. Our mechanical designers were set the task of creating a product that would allow tool-free changeover. The design also needed to be able to integrate with both the floor-standing MS instruments as well as the bench-top QToF and tandem quadrupole MS systems. The engineers were proud to deliver a final design in 2008 that worked well with all three instruments and, crucially, allowed both UPLC and APGC to be installed together. The next and probably most important phase of development was our efforts to maximize instrument performance. Over the years, several groups in the analytical community have pushed to convert GC methods into LC, often with some sacrifice in performance. However, several analyses still require GC because of compound or matrix chemistry – this was a potentially ripe market, and we imagined APGC as an add-on to LC-MS and LC-MS/MS instruments. Initial data showed that a wide range of compounds could be ionized. We presented our work at various conferences and interest came mainly from the more technical mass spectrometry community. On the quantitative LC-MS/MS instrument of the time, the performance looked good, but for ultra trace analysis of persistent organic pollutants (POPs) the overall system sensitivity was not good enough for the most challenging analyses. Soon after the introduction of APGC, development of the next generation of quantitative mass spectrometer began (later named Xevo TQ-S). One of the key components of this system was the implementation of a new type of T-Wave device – the StepWave. The original T-Wave concept was developed by Kevin Giles (another scientific fellow at Waters) as a fast, high-efficiency collision cell. Giles and MS development scientist David Gordon further developed the technology into an ion sampling/transmission device. In a nutshell, the step-wave is an off-axis ion-funnel that actively separates charged and neutral species, increasing signal and reducing background noise, which enables StepWave technology to sample the gas cloud entering from the sample cone orifice with high efficiency.
Initial work with the Xevo TQ-S with APGC was very promising, with the sensitivity and reproducibility of the system exceeding our expectations. The initial results included much checking of calculations and dilutions to check the sensitivity was real rather than an error! Indeed, the Xevo TQ-S was released in 2010 and the improved results gave APGC further potential to perform applications we had not previously thought possible without using a magnetic sector GC-MS instrument.
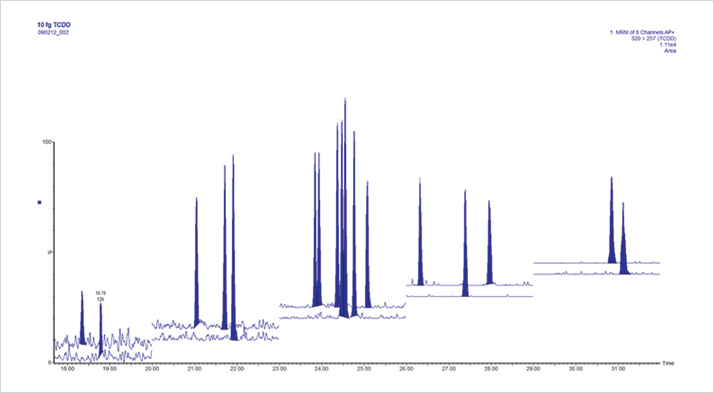
Based on these experiences, we decided we needed to work with key opinion leaders, and a critical meeting occurred in late 2011 with Bert van Bavel, laboratory director at the Man-Technology-Environment Research Centre (MTM), Orebro University, Sweden. Bert has a long history of dioxin and POPs analysis, and saw great promise in the Xevo TQ-S with APGC as a possible replacement for magnetic sector MS, so a collaboration between Waters and MTM was cemented. In 2012, Rainer Malisch and Alexander Kotz from the EU Reference Lab for Dioxins in Food & Feed, Germany, and Wim Traag from RIKILT, The Netherlands, visited Waters in Manchester to run some dioxin and PCB samples. Once again, initial results showed great promise and soon plans were made to perform an extended side-by-side comparison with a magnetic sector MS. Collaboration with key opinion leaders is always an important part of the Waters development process. It not only allows access to skills that are not available in-house but also gives a truly honest appraisal of the performance of the technology. It can sometimes be hard to take an unbiased view of a product that has taken up years of your life! The feedback about the data quality from the Xevo TQ-S with APGC was positive from both groups. The technology started to look very promising as a potential replacement for magnetic sector MS. There are several advantages to this solution. Firstly, the system is easier to use than a magnetic sector, and secondly, the system is flexible, allowing various different analyses to be run on a single platform rather than having a single, dedicated system just for dioxin/furan analysis. After much discussion both internally and externally, Waters launched the technology as a distinct product at the Dioxin 2013 meeting in Daegu, South Korea.
Research and development of APGC technology continues within Waters, both to understand the fundamental principles behind the technology and to fully optimize the technique under different conditions. Acceptance of the technology has been increasing over time and our customers have moved from researchers and opinion leaders working at the leading edge of science to high-throughput commercial laboratories. The pace of development never seems to slow in mass spectrometry, so the next challenge is never far away.
Jody Dunstan is MS Product Manager, Waters Corporation, Wilmslow, UK.
References
- E. C. Horning et al., “New Picogram Detection System Based on a Mass Spectrometer with an External Ionization Source at Atmospheric Pressure”, Anal. Chem. 45 (6), 936–943 (1973). C. N. McEwen, “GC/MS on an LC/MS Instrument Using Atmospheric Pressure Photoionization”, Int. J. Mass. Spec. 259(1-3), 57–64 (2007). C. N. McEwen and R. G. McKay, “A Combination Atmospheric Pressure LC/MS: GC/MS Ion Source: Advantages of Dual AP-LC/MS: GC/MS Instrumentation”, J. Am.Soc. Mass Spectrom. 16(11), 1730–1738 (2005).
