Almost a decade ago, I’d never really given a second thought to how the scope of my analytical research might change in the future. But change it did!
Back in 2010, I was a senior scientist at Graz University. I developed chiral separation methods for active pharmaceutical ingredients, using various high-performance separation techniques, including HPLC, GC, capillary electrophoresis, and capillary electrochromatography – all highly respectable. My former supervisor was retiring, and I was charged with taking over his lessons. I felt that one of those lessons – the analysis of illicit drugs – could do with a little more “spice.” But how?
Hello, officer!
I visited the main police station in Graz and asked if there was any interesting news about abuse of drugs in public parks that I could share with the students; I wanted to ground the lesson in reality. I received a warm welcome – and several interesting stories about dealers – from one police officer. But if it were not for the presence of a second police officer on that day in early March 2010, my scientific field of interest would not have changed so dramatically. This enthusiastic character was excited to ask me if I had heard about “novel psychoactive substances” (NPS) – also known as Legal Highs, Research Compounds, Leisure Drugs, Plant Food, Bird’s Cage Cleaner or Room Odorizers…
The compounds were traded via the Internet or in so-called headshops, he told me, but they were not scheduled by Austrian law, making them a special challenge for the authorities. Because national labs were overloaded, analyses of these tricky samples were often considerably delayed – and dealers were being wrongly imprisoned, given that the compounds could actually be traded legally. The police officer also tipped me off on the name of a compound that came up again and again over the following years: mephedrone (4-methylmethcathinone). After assuring him that I would look into this challenging but exciting situation, I was faced with my first problem. How does one go about procuring even one gram of such compounds? When faced with an unanswerable question, I did what everybody does: I turned to the Internet. A quick search pointed me to a source of mephedrone in the UK
And so I found myself entering into a whole new world with my first purchase of a psychoactive substance. Three days later, my order landed in my mailbox (at home!). I was eager to perform a GC-MS analysis and – somewhat unexpectedly – I discovered that my first sample was of the same purity as denoted on the label. Next, I elucidated the mass spectrum of the compound and, suddenly, I was in a position to help identify mephedrone in samples seized by police.
But I was still intrigued – there was more to learn; mephedrone possesses a chiral center, which encouraged me to develop a chiral separation method to prove that it was being sold as a racemic mixture. I was not surprised when I saw two peaks in my chromatogram – after all, why should such a novel psychoactive substance be traded as a pure enantiomer, when achieving such purity would be a cost-intensive process? That said, at that time, nobody knew which of the two was the eutomer (the enantiomer with the desired pharmacological activity).
As an aside, I was actually very lucky with my first drug purchase; some days later, the law was changed in the UK and mephedrone was banned overnight. As a consequence, many Internet shops were no longer allowed to trade the compound. Meanwhile, a headshop in Graz continued trading mephedrone, apparently serving a long line of customers every weekend. Towards the end of 2010, the first flood of NPS hit the whole of mid-Europe.
A growing collection
I continued to perform analysis for the police, distinguishing between established illicit drugs and NPS that were still legal at the time. At some point, the police showed up with a new powder that did not match the mass spectrum of mephedrone. One of my colleagues was kind enough to record an NMR spectrum, we were now in possession of a brand-new compound that was closely related to mephedrone. The name of this latest designer analogue was 3,4-dimethylmethcathinone (3,4 DMMC) and its very existence hardened my resolve: I would collect as many NPS samples as possible! Specifically, I wanted to know if all such compounds were traded as racemic mixtures and, at the same time, gain knowledge and develop chiral separation methods for these little-known compounds.
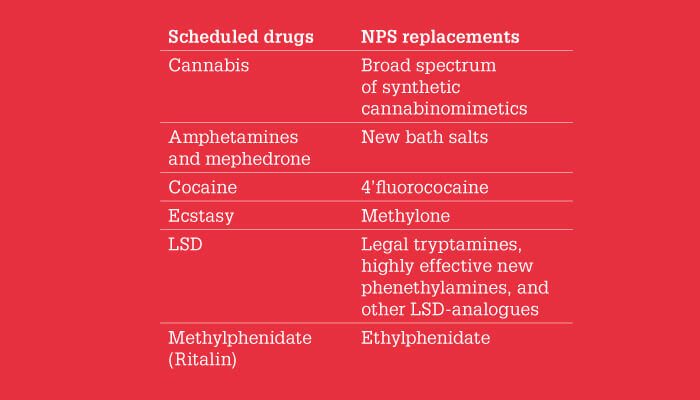
Over time – and with many Internet searches and more than a few purchases (and failed purchases) – I became increasingly familiar with the world of NPS and its (short) history. Essentially, I could see rapidly shifting trends as they happened. After the ban of mephedrone, it was immediately replaced by some closely related compounds, the most prominent of which were the aforementioned 3,4 DMMC and 4-MEC (4-methylethcathinone). Typically, a methyl group was either added or replaced by an ethyl group to circumvent the law. Later, 4-ethylethcathinone (4-EEC) or 4-ethylmethcathinone (4-EMC) was offered instead of 4-methylethcathinone. In parallel, halogens were introduced into the phenyl ring of mephedrone yielding 4-fluoromethcathinone (flephedrone, 4-FMC), and 4-bromomethcathinone (brephedrone, 4-BMC) and 4-chloromethcathinone (clephedrone, 4-CMC). Interestingly, all these compounds were first available in their para-form, followed by meta- and ortho isomers. Methylmethcathinone turned out to exhibit six possible forms: 4-MMC, 3-MMC, and 2-MMC each with two enantiomers.
Eight years later, my NPS collection contained 75 of the approximately 120 known cathinones. All of them were designed to circumvent the law and still little is known about their main effects, side effects and long-term effects. And that’s probably why, seeking some clue as to what to expect, NPS consumers read about the experiences of arguably less-than-trustworthy users on Internet forums…
As another aside, I’ll note that simply growing my collection of hundreds of samples was an interesting endeavor. Notably, prices vary wildly; on Internet platforms 1 g of a compound costs about 20 euros, but through traditional vendors (for example, LGC Pharma) 10 mg costs over 125 euros! In other words, even if I encounter many Internet “scammers” who take my money, but fail to provide the right (or any) substance, overall it’s still cheaper to buy online – assuming you have the analytical capability to check you’ve got the right compound. To that end, after a successful purchase, my samples were identified by GC-MS and, if necessary, by NMR. Interestingly, about 70 percent of my purchases resulted in the correct compound – the other 30 percent? I either got something different or nothing at all. I’d also like to point out that all purchases were made using the “Clearnet” – I absolutely refuse to enter the Darknet!

Where do these huge volumes of so-called “Research chemicals” come from? Well, they can be ordered at the kilogram scale – and they are often manufactured in China. There, hundreds of brilliant chemists wait for orders to synthesize compounds according to a given chemical formula. As far as I know, these people are not always aware of how the compounds will be used down the line.
Novel compounds, novel methods
So, what have I been doing with my collection? I suspect something quite different to most regular buyers! At first, my goal was to build up a GC-MS database of novel psychoactive substance. Next, all chiral compounds were subjected to enantioseparation using various high-performance techniques. Over the years, I’ve tested many different chiral HPLC columns, partially in normal-phase but also in reversed-phase or polar-organic mode. Here, cellulose or amylose-based chiral stationary phases turned out to be a particularly good choice. We were not quite so successful with cyclodextrin-bonded chiral phases; however, the use of ß-cyclodextrin derivatives as mobile phase additives showed positive results both for RP-8 and RP-18 phases (1).
With gas chromatography, the main emphasis was on indirect chiral separation. After sample pretreatment with different chiral derivatization agents, several racemates were resolved as their diastereomeric pairs on a common HP-5 capillary (2) (3).
Supercritical fluid chromatography (SFC) also turned out to be a powerful alternative for the separation of new designer drugs. We published some results of this work with another research group (4).
Besides chromatography, we also developed chiral separation methods for capillary electrophoresis (CE) – a technique with a 25-year history in our lab. We tested several chiral selectors as additives to the background electrolyte. The use of cyclodextrin derivatives as well native ß-cyclodextrin was successful for various chiral compound classes, such as cathinones, amphetamines, benzofuries, thiophenes, phenidine, and phenidate derivatives. Furthermore, the use of a capillary packed with a cellulose-based chiral selector ended up with successful results; once again, this was done with another research group (5).
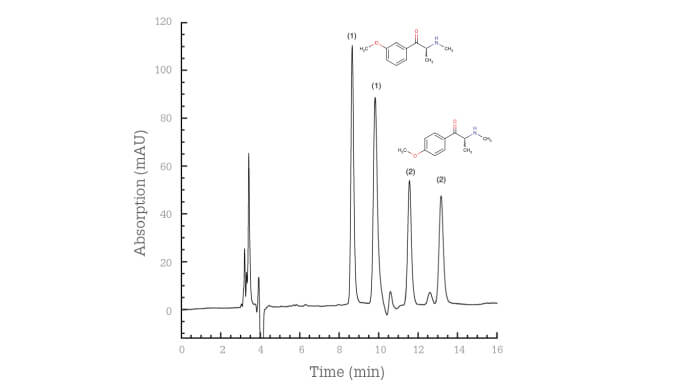
To date, we’ve been able to resolve the enantiomers of more than 100 of these compounds using HPLC (6) (7) (8) and CE (9) (10) (11). When compared with chiral GC-MS analysis, chiral separation with both techniques offers an additional benefit: we’re able to differentiate between ortho- meta- and para-forms because of the different retention/migration times and chiral separation factors of the enantiomer pairs.
(Unexpected) NPS trends
In addition to the prominent chemical compound class of cathinones, native amphetamine and N-methamphetamine (both prohibited) also arrived on the scene with slight alterations; the first derivative showed up in 2011 as 4-fluoroamphetamine (4-FA), followed by 3-fluoroamphetamine (3-FA) and 2-fluoroamphetamine (2-FA). Later, 4-chloroamphetamine (4-CA) and 4-methylamphetamine (4-MA) were traded. Now, after methamphetamines, ethamphetamines are available.
The concept of introducing a beta-keto function to convert an amphetamine into a cathinone led to similar attempts in other classes. Ecstasy analogues are one prominent example: MDMA was replaced by MDMC (methylone). Other closely related substance classes also became very popular: benzofuries, tryptamines, and ketamines. Obviously, the growing number of new compounds reported to the European Monitoring Centre for Drugs and Drug Addiction each year was hard to keep track of.
Over the years, I’ve found myself in a rather interdisciplinary network. Medical doctors ask about the name and structure of “bath salts” so that they can attempt to estimate their effects. Police and judges ask whether a certain new compound is scheduled or not. And I exchange the current “state of the art” with local forensic chemists periodically.
When the first compounds flooded Austria in 2010, I was sure that, eight years later, the majority of drug users would have switched to NPS instead of established drugs in a bid to avoid legal consequences. But, surprisingly, after the initial hype, we’ve seen a significant decline in official consumption of NPS – at least in Austria. The trend may be connected with the Austrian law on NPS, which came into force in 2012. As possession of NPS is allowed (and thus not prosecuted), it is difficult to estimate how many drug consumers actually joined the NPS revolution; despite a significant decrease in drug seizures by police, NPS use is still recorded at Austrian drug outpatient departments. In contrary, in Germany all NPS were added to the Betäubungsmittelgesetz – Narcotics Law – but, interestingly, a special law – Neue-psychoaktive-Stoffe-Gesetz (NpSG) also came into force in late 2016. Other countries, such as Switzerland or the UK, do not have special laws for NPS; instead, NPS are added to existing law (Single Convention 1961).
Given that any classic scheduled drug can be replaced by NPS nowadays, I’m surprised that they aren’t more numerous.
More recently – in 2017 and 2018 – the four hot topics in the synthetic drug world have been: i) synthetic cannabinoids that mimic delta-9-THC present in cannabis, ii) cathinones as the new bath salts, iii) new benzodiazepines – a totally new substance class to replace commonly available benzodiazepines, but without prescription, and, finally iv) dangerous fentanyl derivatives – dubbed “Opiates 2.0” – which have resulted in several fatalities.
The consumption of cannabis (or cannabinomimetics) is still the most popular form of substance (ab)use. Unsurprisingly then, cannabinomimetics are very popular right now – and are being produced from very different chemical substance classes. For consumers, these NPS offer several advantages: they are odorless crystals or liquids that can also be used with electronic devices such as e-cigarettes or e-shishas, and, compared with marijuana, much smaller amounts are sufficient to take effect. They are also more easily smuggled into prisons.
Another trend is the rapidly rising prevalence of cannabidiol-hemp, which is difficult to differentiate from marijuana. When hemp contains less than 0.3 percent delta-9-THC and belongs to the EU certified catalogue of brands, it is legal – but how can we quickly clarify this in the street? It seems to me that the cultivation of cannabidiol-hemp may be a targeted attempt to confuse authorities; after all, it is not so difficult to cultivate a small amount of drug-hemp hidden in a big plantation of cannabidiol-hemp…
The similarity between cannabidiol-hemp and drug-hemp also makes honest quality control problematic. To that end, a well-known vendor of HPLC-UV instrumentation now offers a specially adapted system – the “Cannabis Analyzer for Potency,” which is able to determine several important cannabinoids in a single HPLC run.
Joining the good fight
GC-MS remains the most advantageous approach when it comes to the detection and identification of NPS. As most of the compounds are volatile, there is no need to undergo derivatization prior to analysis. Today, a database of designer drugs containing up to 26,500 mass spectra is commercially available.
My personal hope is for forensic analytical scientists to start working together much more closely – not only locally but also by building up an international network that can enable even more interdisciplinary collaboration. The goal for all scientists should be the same: to lend their clever minds and skills to the battle against the (perhaps hopeless) fight against drugs. NPS should never be regarded as “safe” – no matter what consumers think; after all, there is very little knowledge and few or no clinical studies in most cases. And clearly, given their novelty, there is no information regarding negative health effects from long-term exposure or dependence.
The abundance of emerging compound classes – despite the apparent official lull – is proof that there is no reason to give the all-clear on NPS just yet. Analytical chemists still have a vital role to play.
References
- M Taschwer, Y Seidl, S Mohr and MG Schmid, “Chiral separation of cathinone and amphetamine derivatives by HPLC/UV using sulfated ß-cyclodextrin as chiral mobile phase additive”, Chirality, 26, 411–418 (2014). DOI: 10.1002/chir.22341. S Mohr, JA Weiß, J Spreitz , MG Schmid, “Chiral separation of new cathinone- and amphetamine-related designer drugs by gas chromatography-mass spectrometry using trifluoroacetyl-L-prolyl chloride as chiral derivatization reagent”, J Chromatogr A, 1269, 352–359 (2012). JAWeiß, M Taschwer, S Mohr and MG Schmid, “Indirect chiral separation of new recreational drugs by gas chromatography-mass spectrometry using trifluoroacetyl-L-prolyl chloride as chiral derivatization reagent”, Chirality, 27, 211–215 (2015). K Kalíková, M Martínková, MG Schmid and E Tesařová, “Cellulose tris-(3,5-dimethylphenylcarbamate)-based chiral stationary phase for the enantioseparation of drugs in supercritical fluid chromatography: comparison with HPLC”, J Sep Sci, 41, 1471–1478 (2018). Z Aturki, MG Schmid, B Chankvetadze and S Fanali, “Enantiomeric separation of new cathinone derivatives designer drugs by capillary electrochromatography using a chiral stationary phase, based on amylose tris(5-chloro-2-methylphenylcarbamate)”, Electrophoresis, 35, 3242–3249 (2014). D Albalsa et al, “Chiral separations of cathinone and amphetamine-derivatives: Comparative study between capillary electrochromatography, supercritical fluid chromatography and three liquid chromatographic modes”, J Pharm Biomed Anal, 121, 232–243 (2016). M Taschwer, J Grascher and MG Schmid, “Development of an enantioseparation method for novel psychoactive drugs by HPLC using a Lux1 Cellulose-2 column in polar organic phase mode”, Forensic Sci Int, 270, 232–240 (2017). K Kadkhodaei, L Forcher, and MG Schmid, “Separation of enantiomers of new psychoactive substances by high-performance liquid chromatography”, J Sep Sci, 41,1274–1286 (2018). S Mohr, S Pilaj, and MG Schmid, “Chiral separation of cathinone derivatives used as recreational drugs by cyclodextrin-modified capillary electrophoresis”, Electrophoresis, 33,1624–1630 (2012). M Taschwer, MG Hofer and MG Schmid, “Enantioseparation of benzofurys and other novel psychoactive compounds by CE and sulfobutylether β-cyclodextrin as chiral selector added to the BGE”, Electrophoresis, 35, 2793-2799 (2014). JS Hägele and MG Schmid, “Enantiomeric separation of novel psychoactive substances by capillary electrophoresis using (+)‐18‐crown‐6‐ tetracarboxylic acid as chiral selector”, Chirality, 30, 1019–1026, (2018). DOI: 10.1002/chir.22981