Antibody drug conjugates (ADCs) are a class of therapeutic molecules consisting of three primary components: a monoclonal antibody (mAb), a cytotoxic payload (toxic drug), and a linker molecule connecting the antibody and payload.
mAbs, which are highly specific and selective, serve as the targeting element in ADCs. The antibodies are designed to recognize and bind to specific antigens present on the surface of cancer cells, or other diseased cells. Once the antibody binds to its target, the ADC is internalized by the cell through endocytosis. The cytotoxic payload, often a potent chemotherapeutic agent, is then released inside the target cell due to the degradation of the linker in the cellular environment. This payload exerts its toxic effects, inducing cell death and thereby contributing to the therapeutic efficacy of the ADC.
The rationale behind ADCs lies in their ability to deliver potent cytotoxic drugs selectively to diseased cells while minimizing damage to healthy surrounding tissues. This targeted approach aims to enhance the therapeutic index, maximizing the drug’s efficacy against the disease while minimizing adverse effects on normal cells and tissues.
ADC analysis: not as easy as ABC
One major challenge of ADC analysis lies in the diversity of the three components comprising the ADC. Each of these elements introduces analytical complexities that demand specialized techniques for comprehensive characterization.
Firstly, the heterogeneity of mAbs, arising from post-translational modifications (such as glycosylation and oxidation), necessitates sophisticated analytical methods, such as liquid chromatography-mass spectrometry.
The cytotoxic payload is usually a potent small-molecule drug – often exhibiting a complex structure – but each with varying chemical properties. Analytical methods such as MS and nuclear magnetic resonance (NMR) are examples of techniques that can be used to identify and quantify the payload, ensuring batch-to-batch consistency and adherence to regulatory specifications. Finally, the linker, which connects the antibody and payload, poses challenges in terms of stability and homogeneity. Analyzing the linker’s integrity under various conditions can be performed using LC-MS and NMR. Multiple conjugation sites on the antibody, variations in drug-to-antibody ratios, and potential modifications during the manufacturing process all contribute further to the challenges of analyzing ADCs.
Overall, the analytical challenges associated with ADCs demand a combination of advanced techniques to characterize and quantify the diverse components, ensuring the safety, efficacy, and quality of these complex biopharmaceuticals. Ongoing research aims to address these challenges and further refine analytical methods for robust characterization of ADCs.
Advanced Drugs with Advanced Analytical Requirements
Antibody drug conjugates (ADCs) add several additional layers of complexity, but the promise of a “magic bullet” – and some notable commercial successes – have reinvigorated the field and demanded solutions tailored to the specific needs of ADC development and manufacture.
Here, Agilent’s Andreas Mielcarek explores the analytical solutions powering the next wave of ADCs.
Decoding the ADC Puzzle with Agilent's Analytical Arsenal
Diving into the world of antibody-drug conjugates (ADCs) feels a bit like stepping into a sci-fi novel. These “magic bullets” are designed to zero in on cancer cells with the precision of a heat-seeking missile, delivering their potent payload directly where it is needed most. For those of us getting our feet wet in biopharma analysis, the allure of ADCs is matched only by their complexity.
Size Matters: SEC for Aggregates
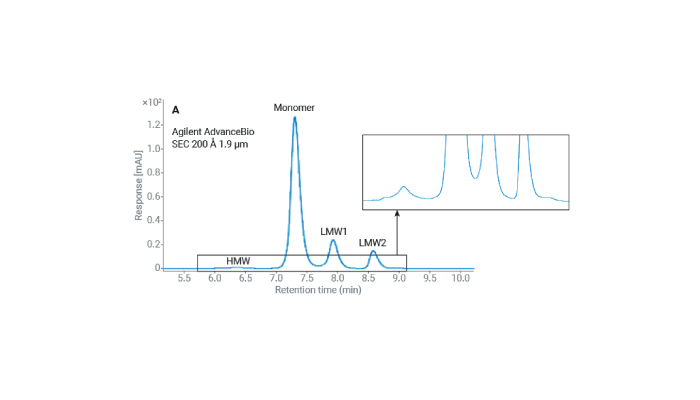
First up, let’s talk about size exclusion chromatography (SEC). If you’re picturing tiny particles weaving through a microscopic maze, you’re not far off. SEC is all about separating the big guys from the small fry in our ADC mixtures. Those high molecular weight (HMW) aggregates? We need to keep an eye on them because they can throw a wrench in the works, potentially triggering unwanted immune responses. Agilent’s AdvanceBio SEC columns are like the elite athletes of the SEC world, offering sharp turns (peaks) and minimal bumping into walls (secondary interactions). And that means you get a crystal-clear view of what’s what in your ADC concoction.
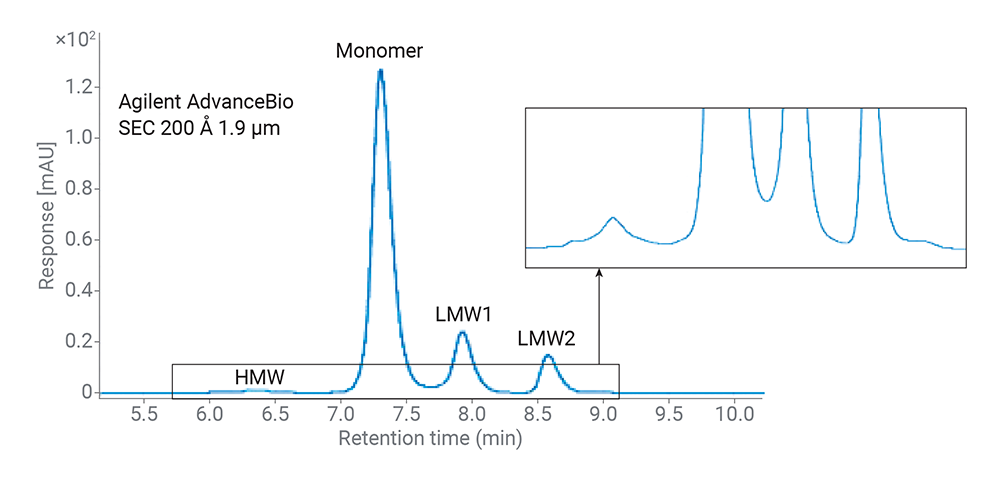
Figure 1. Size exclusion chromatograms of SigmaMAb (mixed with its F(ab’)2 and Fc fragments) using 4.6 × 300 mm SEC columns running with 50 mM sodium phosphate, 200 mM NaCl, pH 7.0 at 0.35 mL/min.
© Agilent Technologies, Inc.
Reproduced with Permission, Courtesy of Agilent Technologies, Inc.
Sticking Together or Sliding Apart: HIC for DAR
Next on our tour is hydrophobic interaction chromatography (HIC). Imagine your ADCs having a pool party where the hydrophobic ones – those that fear water – tend to clump together. HIC is the bouncer at this party, deciding who gets to stay in the pool and who needs to leave, based on how hydrophobic they are. This is crucial for figuring out the drug-to-antibody ratio (DAR), which is important for ensuring your ADC hits the therapeutic sweet spot. The Agilent 1260 Infinity II Prime Bio LC, with its ability to handle the mix of salty buffers and organic modifiers like isopropanol, makes this task a breeze.
Shedding Light with UV-Vis Spectroscopy
Then there’s the Agilent Cary 3500 UV-Vis Multicell spectrophotometer, a gadget so advanced it feels like it belongs in a lab from the future. This tool lets you watch how your ADCs react to different temperatures; it’s like having thermal goggles that show you how your ADCs start to stick together or fall apart as things heat up, helping you measure both DAR and how likely they are to aggregate. And remember: such insights can make or break the success of an ADC.
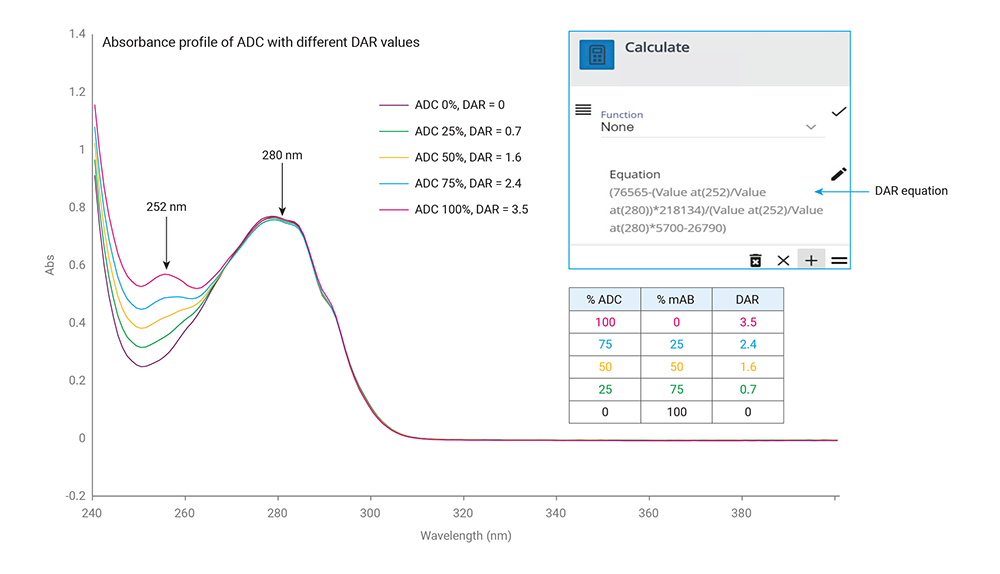
Figure 2. UV-Vis spectra of the ADC and unconjugated antibody mixtures with different average DAR values.
© Agilent Technologies, Inc.
Reproduced with Permission, Courtesy of Agilent Technologies, Inc.
HPLC-MS: The Detective Work
Now, for a bit of detective work with high-performance liquid chromatography-mass spectrometry (HPLC-MS). The super-sleuth of the lab, HPLC-MS is able to pick out the tiniest clues about the ADCs’ structure and payload. Whether it’s identifying where the drug is hitching a ride on the antibody or understanding the payload distribution, HPLC-MS from Agilent gives you the detailed scoop, layer by layer.
Wrapping It Up
For those of us starting in biopharma analysis, the road to understanding and analyzing ADCs might seem daunting. But with Agilent’s arsenal of analytical equipment, guided by human expertise, it feels a lot less like venturing into unknown territory and more like embarking on an exciting adventure. The SEC, HIC, UV-Vis, and HPLC-MS tools we talked about are just the tip of the iceberg of what Agilent offers, but they are a solid foundation for anyone looking to make their mark in analyzing these incredible “magic bullets.”