
Detection of nucleic acids in biological fluids is typically hindered by lengthy preparative procedures and the need for complex instrumentation. However, a new nucleic acid-detecting sensor developed by a team led by Erkin Şeker could potentially filter and detect in one go. The ‘gold sponge’ sensor can sieve out debris in biological samples allowing passage only to target DNA fragments, which may obviate the need for a separate purification step. The team believes that after some optimization of the initial prototype, the device could allow the sensitive, point-of-care molecular detection of DNA in complex biological samples, such as serum from whole blood (1). We caught up with Şeker, assistant professor of electrical and computer engineering at UC Davis, to find out more.
Why are complex biological fluids typically so problematic for sensors?
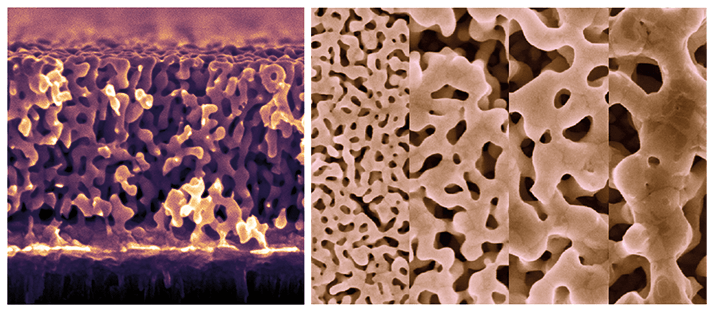
Biological fluids contain large proteins that would normally block-up other sensors (a problem known as biofouling). These large biomolecules adsorb non-specifically on the electrodes surface and prevent the transport of smaller target molecules. Normal electrode-based sensors for the detection of DNA often require external anti-biofouling coatings, but our gold sensor is composed of a network of nano-sized pores, so biological debris, such as proteins, don’t get through, but the nucleic acids can enter easily.Why use nanoporous gold (np-Au)?
Nanoporous gold has a multitude of desirable features, such as high surface area to volume ratio, ease of thiol functionalization, compatibility with conventional microfabrication techniques, and tunable morphology. From a scientific research point of view, it is a great material to study structure-property relationships in a wide range of fields. Technologically, these features make nanoporous gold an excellent choice for DNA sensing applications and enable facile integration and scalability for point-of-care molecular detection systems. However, I must admit that I was not even thinking about bioanalytical aspects of this material when I first put together the research plan of my faculty application package. My initial (and still on-going) research focus has been on multifunctional biomedical device coating. The entire project on bioanalytical sensors started as a result of a university-wide initiative called UC Davis Research Investments in the Sciences and Engineering (RISE) program.How is the sensor made?
We use a self-assembly process to fabricate the np-Au sensor’s gold surface. We then immobilize DNA ‘capture’ probes into the nano-surface. The np-Au electrodes enable detection of target DNA molecules (10–200 nM) in complex biological media, whereas the sensor with planar gold electrodes performed poorly under the same conditions. Nanoporous gold is microfabrication-compatible and electrically-conductive, it is a versatile material to seamlessly integrate with sensing electronics – a problem for some nanomaterials as they cannot be easily integrated into devices by conventional microfabrication techniques. We hope our work on np-Au DNA sensors can serve as a guide for developing nucleic acid sensors using various other novel nanostructured materials.What was the most challenging aspect?
Well, first I needed to learn some electrochemistry! And the main challenge was developing an electrochemical protocol that maintains a delicate balance between reaction- and mass transport-limitations, which directly dictate the sensor performance. Most redox markers mainly reacted with the top portion of nanoporous gold films even before diffusing into the pores to make use of that entire surface area underneath the first layer of pores. We (especially the key researchers, Pallavi Daggumati and Zimple Matharu) developed techniques to address these issues – and we were surprised by how well the variations in nanomorphology allowed for the control of the dynamic range of the sensor via influencing the molecular transport and target capture kinetics.What’s next?
We are about to start evaluating the performance of a np-Au-based sensor in detecting microbes in blood (in collaboration with research from UC Davis School of Medicine and School of Veterinary Medicine). We also have some spin-off ideas that should further assist analytical sample preparation – hopefully we’ll share these results with the biosensor community in the near future. There is a race to develop biosensors that are more sensitive, cheaper, and faster. Though these efforts are commendable, I think the fundamental science behind how the nanostructure dictates sensor performance is very important. We are grateful that the recent National Science Foundation (NSF) award will fund us in continuing to pursue this fundamental question.References
- P Daggumati et al., “Biofouling-resilient nanoporous gold electrodes for DNA sensing”, Anal Chem 87 (17), 8618–8622 (2015).




