Lipids represent the most diverse set of molecules in human biology, with extremely heterogeneous underlying chemistry. For a long time, lipids were largely overlooked in research – but it has become increasingly clear that they have a significant role to play in human health and disease. As analytical tools have become more advanced over the years, we have seen the lipidomics field boom. However, there are still many questions about the exact role this huge diversity of compounds plays in the body.
High-resolution ion mobility MS (HRIM-MS) offers researchers an exciting insight into the structural details of lipids and the impact of altered metabolite pathways on the pathogenesis of many diseases. Measuring such alterations and understanding the pathways involved is crucial to fully understanding cellular metabolism. Here, Kim Ekroos takes us through his work in the field of lipidomics and explains how an emerging HRIM-MS technique based on structures for lossless ion manipulation (SLIM) technology is enabling him to dissect the metabolic glycosphingolipid map in Parkinson’s disease (PD).

I have been working in lipidomics for over 20 years now. After receiving my PhD in biology from the Technical University in Dresden, Germany, in 2003, I continued to work in lipidomics, both in academic research and industry – with CROs, pharmaceutical companies, and eventually running my own business. My expertise includes high-throughput technologies for the accurate assessment of lipidomes enabled by advanced MS, automation, and software tools focusing on the discovery of diagnostic biomarkers for clinical applications. I am pleased that the work I do has had a great impact on the development of the field as a whole, enabling the discovery of the first lipidomics-based biomarkers in clinical diagnostics, for example. I am also a co-founder of the Lipidomics Standards Initiative and President of the International Lipidomics Society.
I was aware that Richard Smith of Pacific Northwest National Laboratory had been working on SLIM because I was also involved in developing another ion mobility technology at the same time. I knew something was being created but not much more than that. Then, a few years back, I was attending a lipidomics meeting in Singapore when I met Melissa Sherman – CEO of MOBILion Systems. We started discussing the fact they were commercializing Richard’s SLIM technology and how that could beneficially serve the lipid community. It was really good to meet with her and find out more about this new ion mobility technology – I immediately saw its potential to provide a much needed analytical option to advance lipidomics research. Our initial work on brain ganglioside measurements proves this; we can generate much more in-depth and quantitative details from our analysis with HRIM-MS.
As part of my work with MOBILion, I helped establish a collaboration within our industry networks that aimed to identify alterations in the metabolism of selective glycosphingolipids in specific brain regions that contribute to the early onset and progression of PD. Our international team includes the research group of Shane Ellis at the University of Wollongong in Australia, Ron Heeren from the Maastricht MultiModal Molecular Imaging Institute (M4I) in the Netherlands, and Nathan Hatcher, a principal scientist at the Department of Neuroscience at Merck. I play a consulting role in the project and it is great to be able to work with such a strong, global network.
Glycosphingolipids are natural cellular fats and part of the PD epidemiology. They are components of cellular membranes that fulfil multiple functional roles, from cell structure and transport to signaling. However, the contribution of glycosphingolipids to PD is not yet fully understood. Our research builds on several technical developments made by the group at M4I in the fields of MS imaging and lipid analysis. And it includes the use of MOBILion’s SLIM-based HRIM instrument to identify alterations in the metabolism of selective glycosphingolipids in specific brain regions that contribute to early PD onset and accelerated progression rates.
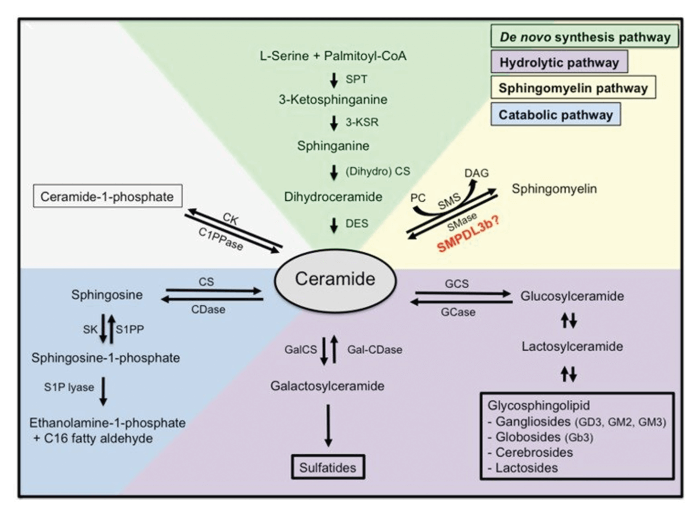
HRIM-MS is helping us to finally look deeper into more complex samples; for example, looking at glycosphingolipids in selective brain regions which have not been well described before. Mutations in the GBA1 gene – the most prevalent genetic risk factor for PD – results in accumulation of glucosylceramide and glucosylsphingosine. However, we do not know the breadth of alterations in glycosphingolipids and how this contributes to PD. We are now combining MS imaging with stable mass isotope precursor labeling methods that allow us to track the synthesis and breakdown rates of glycosphingolipids in different brain regions, in real-time, as well as using the HRIM-MS technology to study how larger, more complex glycosphingolipids are altered in PD.
In October 2020, we were really thrilled to have been awarded a grant from The Michael J Fox Foundation and its partner, the Shake It Up Australia Foundation. Their support means we can now run experiments that have never been done before – identifying the ways in which glycosphingolipid metabolism can be restored in PD. With the other organizations involved in the project, we are looking at matter in the brain and how that is dynamically changing. When we add stable mass isotope precursors to cells – in this case to mice – they are incorporated into the lipid metabolism. Clearly, the labeled precursor has a unique mass, so we can distinguish between the endogenous unlabeled and newly labeled lipids. In this way, we can carefully track the turnover rate of newly synthesized glycosphingolipids, allowing us to extract the properties of sphingolipid metabolism in the greatest detail. In combination with imaging, this not only allows us to pinpoint potential underlying metabolic glycosphingolipid defects in PD but also to localize where in the brain this occurs. We are using the most advanced MS imaging techniques from Shane Ellis’ lab, enabling us to carry out in-depth identification of glycosphingolipids and other lipids and correlate this to the properties of sphingolipid metabolism.
HRIM-MS provides a level of detail on really complex glycosphingolipids that is difficult to access with other technologies. Importantly, there are many different isomeric species, which cannot be separated by MS alone. Other separation methods have been used to measure glycosphingolipids, including thin layer chromatography and hydrophilic interaction chromatography (HILIC) – but they are not only laborious but also insufficient to fully separate the very complex glycosphingolipid kingdom.
By using HRIM-MS technology, we’ve calculated that we can obtain a fifteen-fold gain in throughput – a significant reduction in how much time we spend on analysis!
At the same time, we have higher selectivity and are able to separate isomers; something that we are not able to do with reversed phase or thin layer chromatography – and not completely with HILIC either. Crucially, we do not sacrifice quality with HRIM-MS; rather, we are able to precisely quantify all monitored lipid species. In this respect, it is truly bringing something new to the table – opening up a new level of detail in our analyses. And that has also allowed us to “think” further.
If we look at what is done in clinics or in the pharmaceutical industry today, LC is often found in front of MS analysis. However, LC is typically complicated, brings issues to the measurement, and causes difficulties in the whole workflow. In HRIM-MS separations, 13 meters of path length (in a component that is around 35 x 45 cm!) allows the separation of molecules that have very minor differences. Ion mobility separations are also based on fundamental principles that differ from LC; several characteristics of ionized molecules – size, charge, shape, and structure – come into play at once; in short, HRIM-MS can separate and identify molecules that other instruments fail to detect, while providing a deeper level of structural information.
HRIM-MS represents an opportunity to work with a truly new technique – the benefits of which are reflected in our results to date. And so, I would love to see a reduced dependency on LC… Let’s simply get rid of that unnecessary complexity!




