Therapeutic monoclonal antibodies (mAbs) are proven treatments for a wide range of conditions – from autoimmune diseases to cancer. Their unique structure makes them highly target specific, so fewer side effects are experienced compared with traditional small-molecule treatments. mAb treatments also benefit from a longer half-life – meaning longer-lasting effects.
Nevertheless, as with most treatments, mAbs also pose challenges. Suboptimal exposure for patients is one such challenge, as treatments are generally based on standard doses. If a patient is underdosed, then the treatment may be ineffective – and some patients can develop anti-drug antibodies, further reducing the drug’s efficacy (1). Conversely, overdosing can cause problems, too. Dinutuximab, for example, can cause severe pain in patients when overdosed, as it attaches onto GD2 receptors on nerve cells. Beyond these physical effects, mAb treatments are also highly expensive. Therefore, correct dosing is beneficial to reduce treatment cost.
By personalizing therapeutic protein treatments by using the correct dosing for each patient, we could improve outcomes. But how do we know what dose is correct?
By quantifying the levels of mAbs in plasma, we could better optimize the amount delivered. There are already several methods for quantifying mAbs in plasma, but the most common approach relies on enzyme-linked immunosorbent assay (ELISA, 2). ELISA provides rapid analysis and usually requires inexpensive instrumentation to run; however, it is not considered a particularly robust method, as it generally provides an indirect measurement that can be affected by cross-reactions. Additionally, although initial set-up is cheap, the process itself is costly (consumables are expensive).
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) offers many benefits over ELISA. It is a bottom-up method that measures total mAbs. And, unlike ELISA, LC-MS/MS is selective, precise, and allows for multicomponent analysis. However, traditional LC-MS/MS depends on the availability of “signature” peptides – peptides not present in the patient’s own endogenous immunoglobulins. Additionally, although the LC-MS/MS runs themselves can be quick, sample preparation and method development can be time-consuming; many steps, such as reduction, alkylation, and digestion, all require optimization.
Two-dimensional LC with high resolution accurate mass (HRAM) MS is a next generation solution. 2D-LC uses two different modes to boost separation power – ideal for complex biological samples. And, HRAM-MS allows detection of the intact light chain of the T-mAb, a middle-up approach, meaning that laborious sample preparation, including digestion, can be avoided. Coupling 2D-LC with HRAM-MS represents a powerful approach to quantify various T-mAbs irrespective of signature peptide availability with minimal sample preparation.
Overall, 2D-LC with HRAM-MS shares many of the benefits of LC-MS/MS, including similar instrument cost, while offering much shorter method development and sample preparation time. The approach works by measuring the active form of mAbs, rather than total mAbs. This provides a representative measure of the active antibody fraction in the patient’s plasma, which is more reflective of therapeutic response. Additionally, because the entire light chain is detected, there is always a unique peptide sequence to target – removing the need for signature peptides.

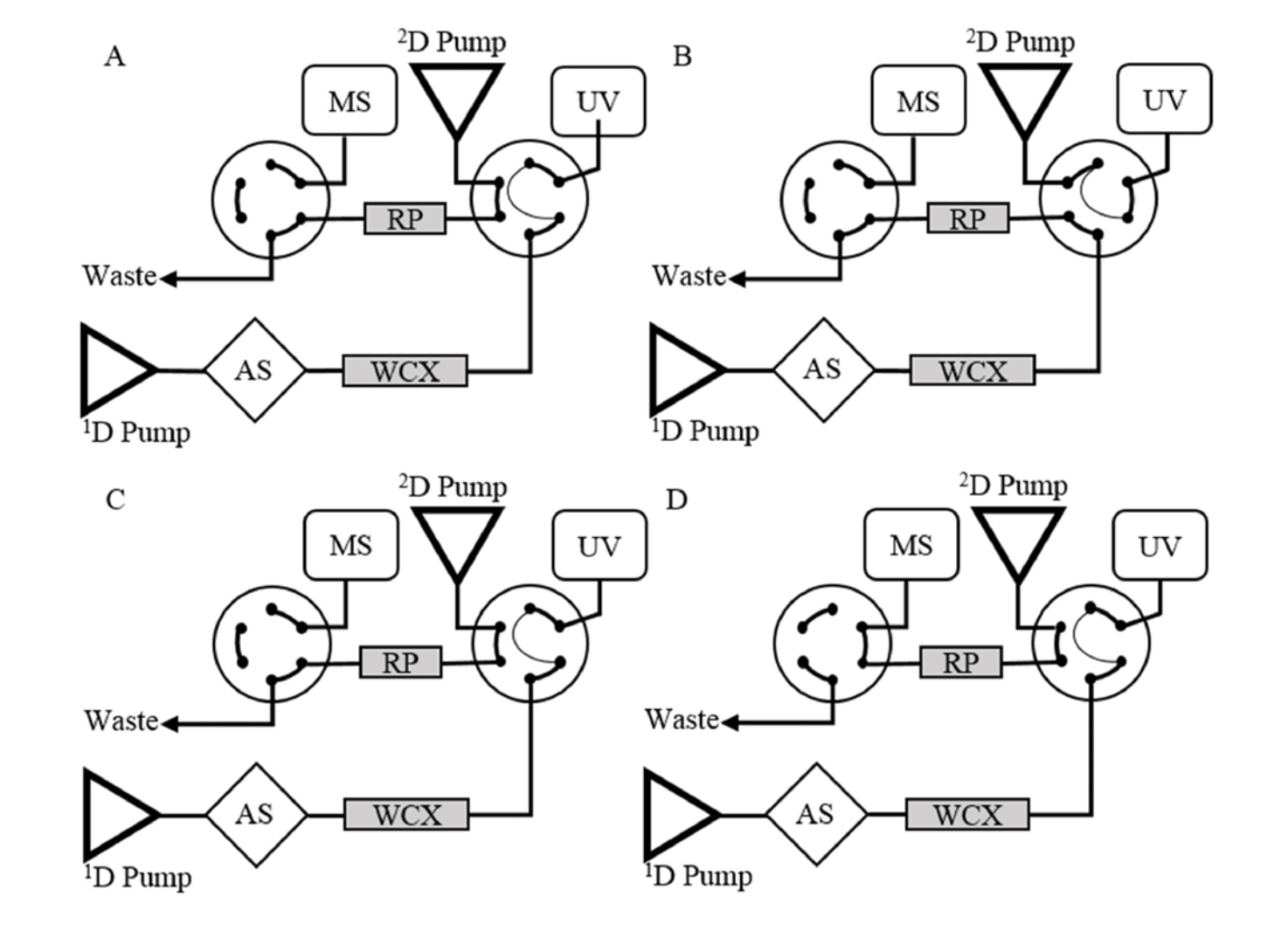
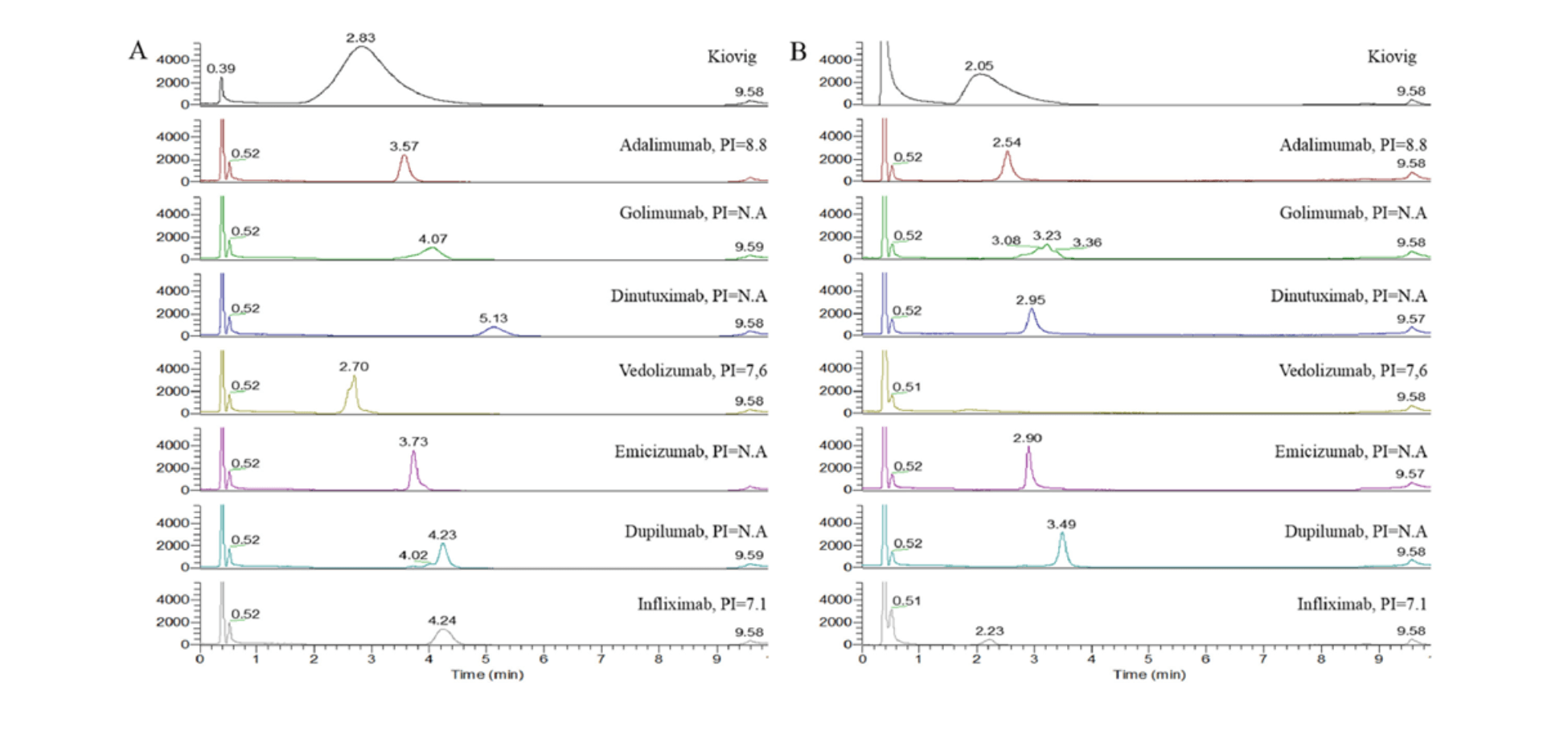
In February 2022, my colleagues from Utrecht University and I validated a 2DLC-HRAM-MS-based method for quantification of Adalimumab in human plasma (3), as a proof of principle and in accordance with European Medicines Agency (EMA) guidelines. To meet the guidelines, method performance parameters such as precision and accuracy need to be below 15 percent for low, medium, and high levels, and below 20 percent relative standard deviation (RSD) and bias for lower limit of quantification (4), which we achieved – a promising sign for 2D-LC HRAM-MS-based mAbs quantification.

Though therapeutic proteins offer revolutionary treatment pathways, they must be dosed at correct levels for the patient to achieve maximum effect. 2D-LC with HRAM-MS provides a sufficiently robust and rapid method for quantifying mAbs.
The technique provides a new way to quickly develop a bioanalytical method for quantifying mAbs in plasma, and will become increasingly important as more mAb therapies become humanized.
References
- Quest Diagnostics, “Adalimumab Drug Level and Anti-drug Antibody for Rheumatic Diseases” (2022). Available at: https://bit.ly/3lLmMMp
- British Society for Immunology, “Enzyme-linked immunosorbent assay (ELISA)” (2022). Available at: https://bit.ly/3GjqjLr
- ME Amrani et al., “Middle-up quantification of therapeutic monoclonal antibodies in human plasma with two dimensional liquid chromatography high resolution mass spectrometry: Adalimumab as a proof of principle,” 1665 (2022). DOI: 10.1016/j.chroma.2022.462840
- EMA, “Guideline on bioanalytical method validation” (2011). Available at: https://bit.ly/38OWVQI




