Suppressor technology was first introduced in 1975. By lowering the conductivity of the mobile phase prior to conductivity detection, it made ion-exchange chromatography more broadly accessible. Now, a team from the Vrije Universiteit Brussel is aiming to take the technology a step further, and in the process talented PhD student Sam Wouters won the Solvay Award for Young Chemists. Wouters and supervisor Sebastiaan Eeltink tell us more.
What prompted you to work on this technology?
As a team, we actually worked not only on the suppressor module but the complete miniaturization of an ion chromatography system. Our aim was to make a portable device allowing for on-line/at-line process monitoring and field analysis – a work in progress!
How is membrane suppressor technology used?
The suppressor is included after the separation column and prior to the detector. In ion chromatography, we try to detect ions using an ionic solution such as NaOH as the mobile phase. As conventional UV detection cannot be used (many ions do not have chromophores) conductivity detection is most frequently applied. However, with so many ions already in the mobile phase it is very difficult to detect “target ions” as the linear range of the detector is very low (most of the range is “used” by the ions present in the mobile phase). We therefore need to use a suppressor to remove these ions.
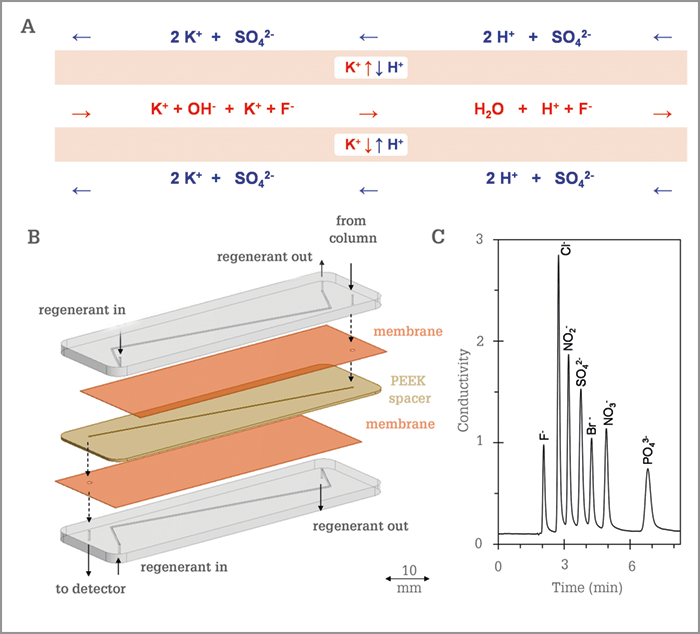
What were the limitations of previous technology?
Early suppressors or “strippers” consisted of large i.d. columns packed with ion-exchange resins, and had limited capacity. There were also problems with mechanical stability of the fiber and with establishing an interface with other system components.
How is your suppressor different?
As shown in Figure 1, we have developed a continuously chemically-regenerated microfluidic membrane suppressor chip for anion-exchange chromatography. When the separation in the column is completed, the analytes and mobile phase (composed of potassium and hydroxide ions) are introduced to the suppressor chip via the eluent microchannel located in a PEEK spacer, that is sandwiched between selective cation-exchange membranes and two thermoplastic chip substrates containing microfluidic channels, which provide the regenerant flow (in this case a diluted sulfuric acid solution). The sulfate counter ions are relatively large and the negatively charged membranes repel these ions. Protons will diffuse through the membrane, replacing K+ ions to pair-up with the hydroxide ions and form water, hence reducing the background conductivity and enhancing the signal. Figure 1C shows the potential of the microfluidic suppressor chip for the high-throughput separation of a mixture of seven anions by coupling a microfluidic membrane suppressor chip to a capillary anion-exchange column.
How will this technology help analytical scientists reach their goals?
Ultimately, we hope this chip will allow you to analyze very small volume samples (important for clinical applications) but also establish modules so you can measure at-line and improve process control. There is still work to be done – it needs to be a very robust “plug and play” chip device – but we believe that microchip suppressors could prove useful in a number of fields.




