For many years, FFF remained the playground for a limited number of research groups. Sub-techniques with different types of fields were studied (gravitational/centrifugal, thermal, electric, magnetic), and some of these were used in commercial instrumentation and niche applications. However, only one of the sub-techniques – flow FFF – has seen a breakthrough into the real world – and that was only recent. In flow FFF, a second flow through the porous wall(s) of the channel creates the field for separating the analyte particles. This second or cross flow is perpendicular to the direction of the main flow. The cross flow drives analytes to the accumulation wall, which is covered by an ultrafiltration membrane through which the carrier solution passes while retaining analyte (macro)molecules and particles.
In 1966, J. Calvin Giddings published “A New Separation Concept Based on a Coupling of Concentration and Flow Nonuniformities” in Separation Science (1). His innovative idea suggested transporting a mixture of different analytes through a thin channel using a laminar (Poiseuille) flow of a carrier solution. If you ‘force’ specific analytes into particular flow lines in the channel, using an external field perpendicular to the flow direction, the differences in velocity between the flow lines will separate the analytes much faster than the field itself.

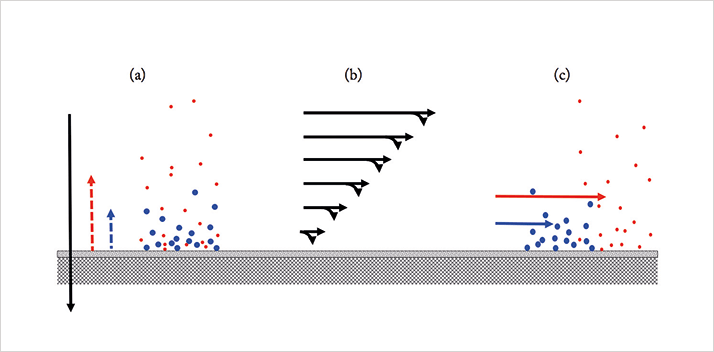
For many years, FFF remained the playground for a limited number of research groups. Sub-techniques with different types of fields were studied (gravitational/centrifugal, thermal, electric, magnetic), and some of these were used in commercial instrumentation and niche applications. However, only one of the sub-techniques – flow FFF – has seen a breakthrough into the real world – and that was only recent. In flow FFF, a second flow through the porous wall(s) of the channel creates the field for separating the analyte particles. This second or cross flow is perpendicular to the direction of the main flow. The cross flow drives analytes to the accumulation wall, which is covered by an ultrafiltration membrane through which the carrier solution passes while retaining analyte (macro)molecules and particles. The Giddings group’s original (“symmetric”) design incorporated a channel with two porous walls for flow FFF (2). Later, Karl-Gustav Wahlund proposed an asymmetrical system, with only one porous wall, to simplify flow control (3). In this asymmetrical flow field-flow fractionation (AF4) mode, the carrier solution is pumped into the channel and splits into the cross flow, passing through the porous wall, and the channel flow transports the analytes to the detector. The accumulation of analyte particles on the wall resulting from the cross flow is counteracted by diffusion, and each analyte ends up in a diffusion layer close to the wall and has a specific characteristic thickness. Because the cross flow is the same for all analyte molecules or particles, the thicknesses of the layers for different analytes is determined purely by their diffusion rate, which is governed by their size.
Figure 1 illustrates the principle of AF4. It shows analyte particles with higher diffusion coefficients enter faster channel flow lines and are the first to elute. AF4, therefore, separates analytes by hydrodynamic size and by the effective size of the molecules/particles in solution.
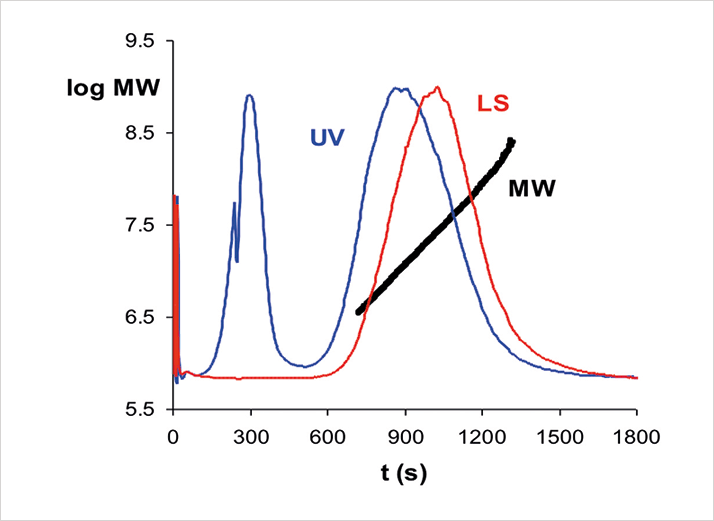
There are a number of alternatives to AF4 for determining size distributions of molecules and particles. But, what are its strengths and weaknesses? One of its often-quoted strengths is that it is a soft separation method, with little surface interaction and shear stress. However, its best asset in my (and Giddings’ [4]) opinion, is its ability to handle a wide range of particle sizes, from those retained by an ultrafiltration membrane, to micrometer-sized solid particles. AF4, therefore, covers the gap adequately between size exclusion chromatography on one hand and various particle-sizing methods on the other. And, the experimental conditions can be adapted easily to fit the expected size of the analytes. Moreover, it is possible to analyze flow-programming samples containing components of varying sizes in one run. Figure 2 shows an example of this from our own laboratory.
AF4 also has weaknesses. It is not a very selective or efficient separation technique. This is because of slow equilibration within the diffusion layer – the main contribution to peak broadening. There is only one remedy to this problem: miniaturizing the channels. Also, there is often a delicate balance between overloading effects and detection sensitivity, because the compounds of interest in the sample solution are highly concentrated in the accumulation layer first, and upon elution highly diluted in the carrier solution.
There has been a noticeably significant increase in the use of AF4 for research and routine analysis over the last few years. So, 50 years after Giddings’ initial ideas, and 40 years after the first experimental demonstration, flow FFF is finally taking off. The slow start was probably due to the lack of suitable instrumentation in the early years. However, several conceptual improvements and innovations in AF4 instrumentation (flow programming options, frit inlet and outlet systems, hollow fiber channels) have since been introduced. But, the big step forward is the availability of more robust and reliable instruments. Another explanation for the increasing interest in AF4 is demand. For example, macromolecular biopharmaceuticals are the future for the pharmaceutical industry; in polymer science, ultra-high molecular weight polymers attract attention; in materials science, chemical technology, and in environmental and health studies, nanoparticles are hot. AF4, therefore, may offer solutions to some of the separation and characterization issues that arise in all of these areas.
In the FFF Hot Seat: Christoph Johann, founder and MD of Wyatt Technology Europe, the company that originally introduced field-flow fractionation (FFF) instrumentation to Europe’s analytical scientists, looks at the history of the technique and shares his involvement with the field.
Taking FFF to the Next Dimension by Thorsten Klein, founder and CEO of Postnova Analytics, Landsberg, Germany.
References
- J.C. Giddings, “A New Separation Concept Based on a Coupling of Concentration and Flow Nonuniformities”, Sep. Science, 1, 123 (1966). J.C. Giddings, F.J.Yeng, M.N.Myers, “Theoretical and Experimental Characterization of Flow Field-Flow Fractionation”, Anal. Chem., 48, 1126 (1976). K.G. Wahlund, J.C. Giddings, “Properties of an Asymmetrical Flow Field-Flow Fractionation Channel Having One Permeable Wall”, Anal. Chem., 59, 1332 (1987). J.C. Giddings, “Field-Flow Fractionation: Extending the Molecular Weight Range of Liquid Chromatography to One Trillion (1012)”, J. Chromatogr. A, 125, 3 (1976).




