- Mass spectrometry imaging could be valuable in the clinic – if existing concerns can be addressed
- MSI allows measurement of small-sized samples and good specimen preservation for additional studies
- It can also be combined with other techniques, such as digital PCR, to make best use of limited biopsy tissue
- Although MSI shows promise for clinical use, standardization efforts are needed, because results remain variable
Until relatively recently, matrix-assisted laser desorption/ionization (MALDI) mass spectrometry imaging (MSI) was restricted to basic science – and it has yet to be widely accepted for clinical research analysis. For MSI to migrate from a research platform to a universally accepted tool, it requires a standardized procedure for everyone using the technique and concerns regarding reproducibility must be addressed. Pathology laboratories increasingly rely on molecular testing that uses genomic technology; emerging molecular technologies, such as MSI, that provide dynamic, untargeted information about the cell’s active state are likely to follow suit in the coming years.
Many of MSI’s features make it ideally suited to clinical research pathology applications. Importantly, it can use the formalin-fixed paraffin-embedded (FFPE) tissue sections routinely collected during hospital care. These specimens maintain excellent tissue morphology and allow the direct collection of both spatial and molecular information. And, because this tissue acquisition method doesn’t require analyte-specific reagents, MSI can even be used for biomarker discovery.
Traditional LC-MS/MS analyses of FFPE tissues require large sample sizes – impractical in the clinical setting – and sample preparation procedures may result in the loss of spatial information. MSI experiments, in contrast, retain the spatial distribution of the multiple molecules detected, because the measurements are conducted on intact tissue sections. One example is MALDI MSI-based tissue typing experiments, in which tissue- or clinically specific molecular profiles are created. MSI’s label-free and nondestructive nature make it an ideal technique for preserving tissue material, such as cancer biopsies, for subsequent analyses.
Despite MSI’s advantages for molecular analysis, the variability of results currently restricts its clinical use. To apply the analytical benefits of MSI to clinical research pathology, the technique’s reproducibility across multiple laboratories and geographical locations must be demonstrated; many basic science laboratories optimize methodologies to their local conditions. A simple way to examine reproducibility is to apply a completely standardized sample preparation and imaging workflow across multiple sites. A recent study did just that when it examined whether several laboratories could achieve reproducible results by analyzing FFPE samples with a standardized MSI workflow (1). The aims of the study were threefold: i) to confirm whether the MSI protocol can maintain spatial resolution across experiments, ii) to confirm application to clinical research samples, and iii) to assess the reproducibility of results across multiple sites.
The study’s authors assumed that trypsin application and digestion were sources of variation and loss of spatial resolution in the MSI of FFPE samples – but results indicated that, when using a standardized workflow, they could discriminate discrete histological features in different tissues, eventually enabling different sites to generate images of similar quality. Using the integrated MSI tissue typing workflow for tryptic peptides from FFPE tissues, specific m/z features were detected in discrete histological features in mouse intestine, human ovarian teratoma, and human squamous cell carcinoma of the lung (see Figure 1).
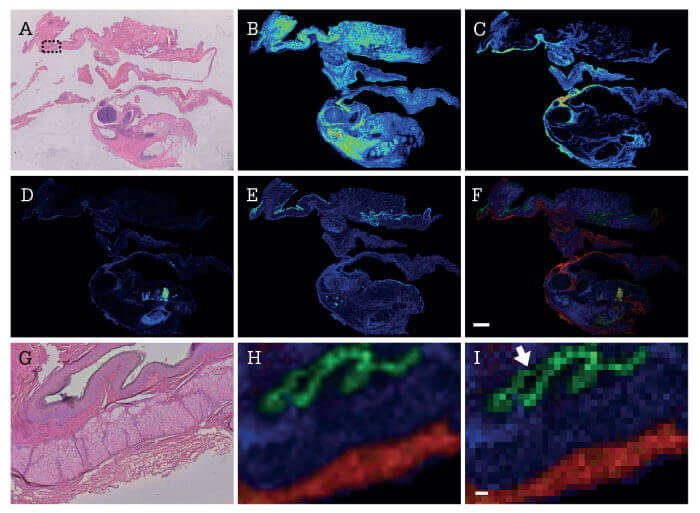
When applied to the multicenter study, the MSI workflow could delineate the same histological features on the mouse intestine across five different sites, demonstrating the technique’s reproducibility when laboratories adhere to stringent sample preparation and standard operating procedures. The study’s results show a reduced likelihood that MSI-based tissue typing-derived classifications will differentiate samples based on technical differences, such as processing origin site, rather than biological or pathological differences – crucial if MSI is to be used as a clinical research tool.
Because clinicians often make crucial decisions based on the results of a wide variety of tests, the non-destructive and tissue sample-preserving aspect of MSI may also benefit personalized medicine. For example, the development of novel therapies targeting oncogenic driver mutations has the potential to improve patient prognosis. Digital polymerase chain reaction (dPCR) is commonly used to reliably detect genetic mutations, and its combination with MSI has great potential for patient stratification. Researchers have demonstrated the ability to carry out MSI and dPCR analyses from the same tissue section (2), which is highly advantageous, given that biopsy material is often limited. This combination of proteomic and genetic analysis in one workflow is made possible by MSI’s noninvasive nature, which has been shown to maintain DNA quality for subsequent analysis.
If MSI is to be adopted for wide-scale clinical research applications, such as diagnostics, its ability to generate the same results from samples collected and measured at different sites is crucial. However, being reproducible is one piece of the puzzle; having a relevant role in the pathologist’s toolbox is another. For example, MSI has been shown to differentiate between adenocarcinoma and squamous cell carcinoma of the lung (3) – a challenging task for traditional immunohistochemistry (IHC) techniques. Additionally, MSI requires only one slide (unlike multi-slide IHC), allowing tissue to be saved for subsequent predictive molecular testing.
The continuing development of mass spectrometers, particularly with respect to improvements in spatial resolution, sensitivity, and sample throughput, is opening the field of MSI to clinical research pathology. The speed and robustness of modern mass spectrometers allows large specimens to be analyzed quickly over large patient cohorts with good spatial resolution – imperative for driving this technique forward in diagnostics, prognosis, and monitoring treatment responses.
References
- A Ly et al., “Site-to-site reproducibility and spatial resolution in MALDI-MSI of peptides from formalin-fixed paraffin-embedded samples”, Proteomics Clin Appl, 13, e1800029 (2019). PMID: 30408343.
- D Kazdal et al., “Digital PCR after MALDI mass spectrometry imaging to combine proteomic mapping and identification of activating mutations in pulmonary adenocarcinoma”, Proteomics Clin Appl, 13, e1800034 (2019). PMID: 30216696.
- M Kriegsmann, “Reliable entity subtyping in non-small cell lung cancer by matrix-assisted laser desorption/ionization imaging mass spectrometry on formalin-fixed paraffin-embedded tissue specimens”, Mol Cell Proteomics, 15, 3081 (2016). PMID: 27473201.




