The standard QC method for mAb aggregate and fragment analysis is size exclusion chromatography (SEC). A new series of 2 micron silica based UHPLC columns with 25 nm pore size can be applied to either increase speed or improve resolution of the separation of antibody fragments, monomers, and dimers.

Experimental:
Columns: TSKgel UP-SW3000, Competitor Protein SEC 200 column
Column size: 4.6 mm ID x 15 cm
Eluent: 100 mmol/L phosphate buffer (pH 6.7) + 100 mmol/L sodium sulfate + 0.05% NaN3
Flow rate: 0.35 mL/min
Temperature: 25 °C
Detection: UV @ 280 nm, micro flow cell
Sample (Calibration):
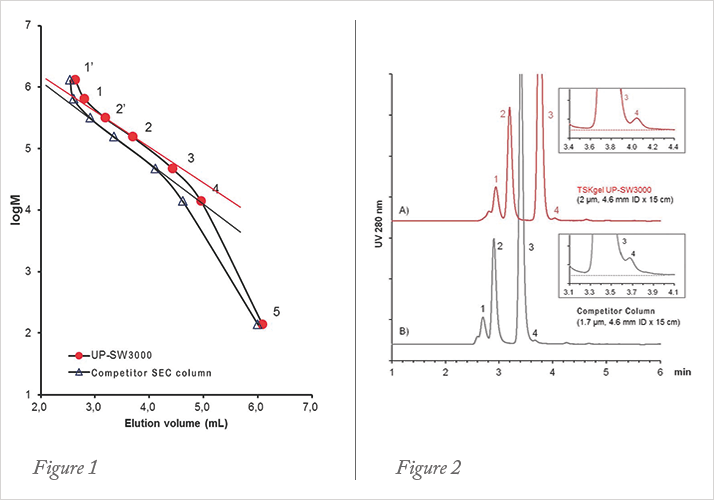
1. thyroglobulin, 640,000 Da (1’ thyroglobulin dimer);
2. γ-globulin, 155,000 Da (2’ γ-globulin dimer);
3. ovalbumin, 47,000 Da;
4. ribonuclease A, 13,700 Da;
5. p-aminobenzoic acid, 137 Da; Inj. Volume 5 μL
Sample (mAb Analysis): therapeutic mAb (mouse-human chimeric); 1: trimer; 2: dimer; 3: monomer; 4: fragment ; Inj. Volume 10 μL
The calibration of TSKgel UP-SW3000 shows a slightly shallower slope in the region of the molecular weight of γ-globulin than the one of a competitor 1.7 micron UHPLC column (figure 1). These differences in the separation range and steepness of the curves are related to a slight difference in pore size (25 nm for TSKgel versus 20 nm for the 1.7 µm material). The separation of an antibody on the new 2 μm packing compared to the competitor column is depicted in figure 2 . The difference in pore sizes results in a slightly better separation in the mass range of antibodies, fragments and aggregates. Based on the wider separation window the resolution is slightly higher with TSKgel UP-SW3000.
TSKgel UP-SW 3000 is ideally suited for the analysis of the aggregate and fragment contents of antibody preparations. It features the same pore size as the renowned TSKgel G3000SWXL column while improving resolution through a smaller particle size. Based on the optimized pore size and the high degree of porosity the resolution in the range of IgG is even superior to a competitive UHPLC column with slightly smaller particle and pore size. www.tosohbioscience.de





