This four-part series examines common issues and questions surrounding the principles, measurements and analysis of DLS data and discusses how to minimize the time required for and increase the accuracy of acquiring and interpreting DLS data during the biotherapeutic development process. In Part Two, we cover the influence of concentration effects and particle interactions on DLS results and provide a roadmap for identifying and distinguishing each type of concentration effect.
A Malvern Instruments' Bioscience Development Initiative
Executive Summary
Dynamic light scattering (DLS) is an analytical technique used to measure the particle size distribution of protein formulations across the oligomer and sub-micron size ranges of approximately 1 nm to 1 µm. The popularity of DLS within the biopharmaceutical industry is a consequence of its wide working size and extended sample concentration ranges, as well as its low volume requirements. With that said, the challenge that remains with the application of DLS to protein therapeutic formulations is centered around data interpretation. In this four-part white paper series, common issues and questions surrounding the principles, measurements and analysis of DLS data are discussed in order to help minimize the time required for and complexity of acquiring and interpreting DLS data that is critical throughout the development process. In this second white paper of the series, we cover the influence of concentration effects and particle interactions on DLS results and provide a roadmap for identifying and distinguishing each type of concentration effect.Dynamic Light Scattering
Dynamic light scattering (DLS) is an analytical technique used within bioapplications to measure particle size distributions across the oligomer and sub-micron size ranges. Light scattered from a solution of particles will fluctuate with time, due to Brownian motion or diffusion. In a DLS measurement, the scattering intensity fluctuations are correlated across small time spans, yielding the distribution of diffusion coefficients from which the particle size distribution is calculated.Historically, DLS measurements have been restricted to the analysis of dilute solutions, with published results at the limit of infinite dilution and unexpected (or unexplainable) concentration effects being cast into the catch-all category of "particle interactions". Modern DLS systems, however, can provide diffusion coefficient measurements of samples at very high concentrations, even to the extent of high turbidity or opalescence, and the widespread use of these systems within the bioformulation market have rendered the generic "particle interactions" rationale unacceptable. In fact, bioformulators now quantitate particle interactions by measurement of the DLS interaction parameter (kD) and the second virial coefficient (B22) and then use these properties to screen biotherapeutic candidates and conditions for subsequent stability.
When exploring the influence of sample concentration on DLS analysis of bioformulations, there are four issues that need to be considered: multiple scattering, restricted diffusion, reversible self-association, and electrostatic repulsion. Each of these effects can be identified during the course of a concentration-dependent DLS experiment.
Concentration Effects
Multiple Scattering As the sample concentration is increased, the probability that a scattered photon will interact with another macromolecule and be rescattered increases (Figure 1). This rescattering effect is called multiple scattering.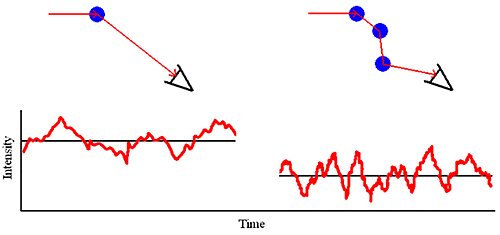
Figure 1: Multiple scattering schematic.
Multiple scattering can easily be identified during the course of a DLS dilution experiment. Symptoms of multiple scattering include the following:
As the sample concentration is increased,
- The scattering intensity will decrease, due to the reduction in the light scattered in the direction of the detector with each rescattering event
- The intercept of the correlation curve will decrease, due to the reduction in measured scattering intensity
- The correlation curve will initially decay faster, but often with a smaller slope, due to the randomness of the multiple scattering events
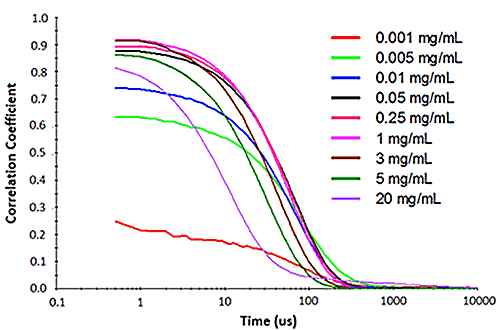
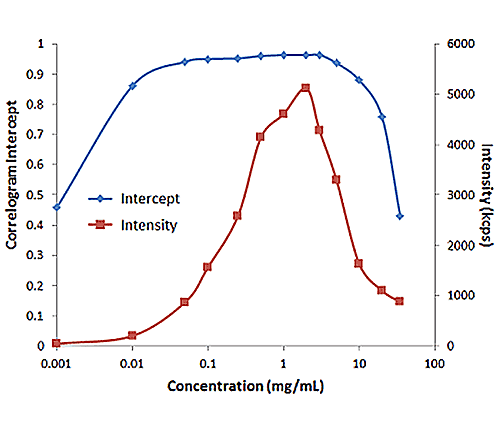
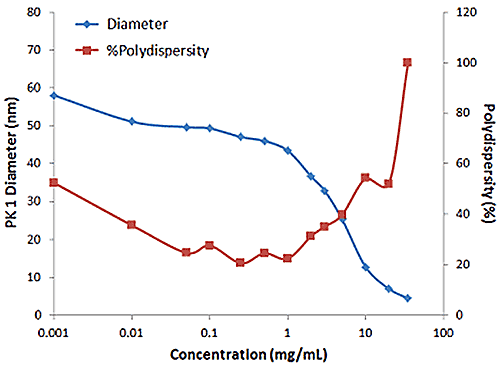
Figure 2: Influence of multiple scattering on DLS results for a PEGylated biotherapeutic.
The concentration at which multiple scattering occurs is dependent upon the size of the particle being measured. Unfortunately, there is no clear delineation between samples exhibiting only single scattering and those exhibiting multiple scattering, but rather a gray area where the magnitude of multiple scattering becomes sufficient to influence subsequent size calculations.
At the diffuse, highly concentrated limit, photons can be considered to undergo a 'random walk' through the sample. According to Pine and Weitz (chapter 16 in "Dynamic Light Scattering: The Method and Some Applications" ed. Wyn Brown, 1993), the diffusive limit of multiple scattering is governed by the photon mean free path, or the distance which an average photon travels in a sample before encountering a scattering particle. Using Mie theory, it is possible to calculate the mean free path when the particle diameter, refractive index of particles and dispersant, laser wavelength, and sample concentration are all known. The mean free path measurement then defines the concentration & size dependent minimum particle separation distance for single scattering samples. Figure 3 shows the size dependence of the maximum sample concentration for single scattering DLS using a 633 nm laser, for aqueous protein systems.
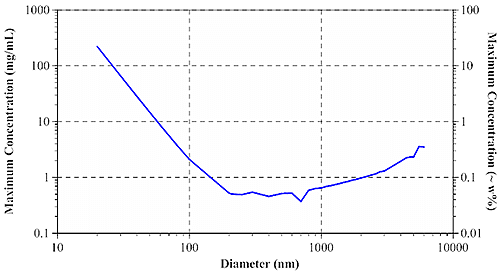
Figure 3: Size dependence of maximum sample concentration for single scattering DLS. For typical protein samples with diameters of < 20 nm, the maximum concentration for single scattering is in excess of 200 mg/mL. For larger aggregates and other biopolymers, the maximum concentration drops off quickly, leveling out at around 0.5 mg/mL or 0.05 w% for particles greater than approximately 200 nm in diameter. Modern backscatter systems such as the Zetasizer Nano can often compensate for multiple scattering effects by measuring near the cell wall, thereby minimizing the distance between the scattering center and the detector. Backscatter systems can increase the maximum concentration for single scattering by as much as 20 fold. With that said, backscatter technology works very well for opalescent or opaque samples, where the scattering intensity is large enough to render flare noise insignificant. For water-clear biosamples however, flaring from the cell wall can be significant - large enough in fact to render cell wall measurements problematic. >> Download the full Application Note as PDF
Malvern provides the materials and biophysical characterization technology and expertise that enables scientists and engineers to investigate, understand and control the properties of dispersed systems. These systems range from proteins and polymers in solution, particle and nanoparticle suspensions and emulsions, through to sprays and aerosols, industrial bulk powders and high concentration slurries. Used at all stages of research, development and manufacturing, Malvern’s instruments provide critical information that helps accelerate research and product development, enhance and maintain product quality and optimize process efficiency. Our products reflect Malvern’s drive to exploit the latest technological innovations. They are used by both industry and academia, in sectors ranging from pharmaceuticals and biopharmaceuticals to bulk chemicals, cement, plastics and polymers, energy and the environment. Malvern systems are used to measure particle size, particle shape, zeta potential, protein charge, molecular weight, mass, size and conformation, rheological properties and for chemical identification, advancing the understanding of dispersed systems across many different industries and applications. www.malvern.com Material relationships http://www.malvern.com/en/ portal@malvern.com





