It’s no coincidence that the human nose is directly over the mouth, nor that there is an “aromatic” class of organic compounds. While smell is not strictly speaking a component of taste, it certainly can have a significant effect. On the quantitative analytical testing side, the Purge and Trap method can extract and concentrate volatile flavor compounds from a liquid sample for subsequent analysis by GC/MS. In this application, a soft drink was tested with purge and trap-GC/MS on a CDS 7000C purge and trap concentrator coupled to PAL RTC 850 System. The results reveal some interesting findings.
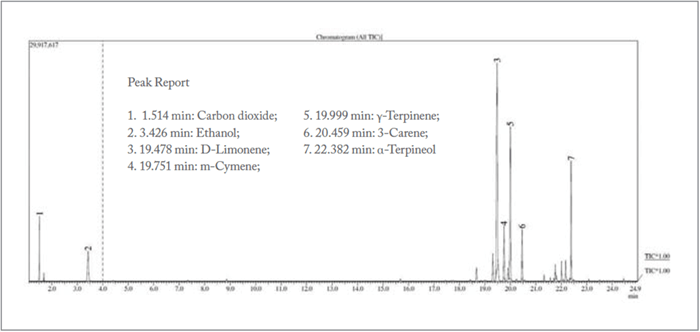
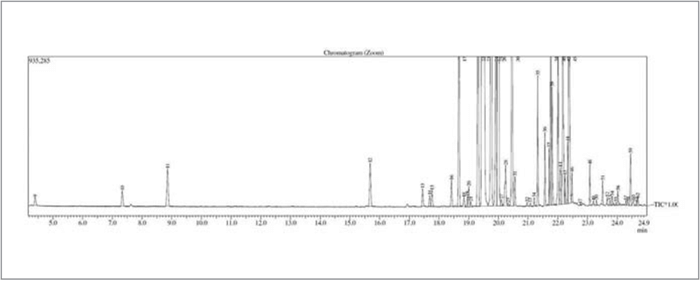
Figure 1 tagged the seven most significant peaks from a soft drink sample. Of particular interest was that ethanol was found at sub hundred ppb level. Typically, beverage manufacturers’ recipes and formulations are trade secrets. Based on general industry knowledge and analytical experience it is highly unlikely that ethanol is added as a formulary compound. Since the sample contained sugar, low-level ethanol is hypothesized to be the result of fermentation. Ethanol is among the compound list, supporting findings by previous researchers and of concern to consumers, although at a very low level. Figure 2 depicts a more detailed look, with more than sixty compounds identified in the purge and trap-GC/MS chromatogram.
The data presented here is a simple capability demonstration. As a routine practice, quantitative P&T-GC/MS is performed in EPA Methods 525 and 8260 for aqueous samples. Potential applications for ethanol content include process monitoring/optimization and comparative sample analysis for diet beverages.
Contact: Carol Byrd +1 610 932 3636
Download the full application note: tas.txp.to/0518/CDS