Remora fish, which spend most of their lives hitching a ride on whales, turtles, sharks and other host animals, are nature’s solution to a tricky problem: stable underwater adhesion to dynamic, highly morphable soft substrates under extreme conditions – all while withstanding drag, fluid turbulence, and tissue regeneration. Could researchers hoping to develop more effective ways of delivering and maintaining drugs and sensors in the gut – protected by a thick, slippery mucosal barrier and with a lining that renews every three days – learn a thing or two from the suckerfish?
“Nature’s had millions of years of R&D – we’d be foolish not to pay attention!” says Giovanni Traverso, associate professor at Massachusetts Institute of Technology, USA, and senior author of a recent study, published in Nature, which demonstrates just that – an adhesion system inspired by remora. “What fascinated us most wasn’t just their grip – but how passive and adaptable it is. Unlike most synthetic adhesives, they don’t use glues or motors. They rely on clever mechanics and microstructures. This made Remora the perfect inspiration for our mechanical underwater soft adhesion system – MUSAS.”

MUSAS uses a combination of shape-memory lamellae and multicompartment suction, mimicking how remoras use their discs. “When exposed to body temperature, the lamellae pop into a preset shape and interlock with the tissue,” says Ziliang (Troy) Kang, the lead author of this study. “Each compartment then forms its own mini vacuum seal that is complementary to each other, which helps the device conform to the surface – whether it’s wrinkled, wet, or healing.” This approach allowed the researchers to avoid the need for glues or external actuation.
The researchers used a range of microscopy imaging and bioanalytical tools to assess the system’s performance in vitro and in over 58 pigs and eight fish. They found that MUSAS could adhere securely to a wide range of soft substances with varying roughness, stiffness, and structural integrity – across extreme pH, moisture levels, and even tissue movement. The team also achieved an adhesion-force-to-weight ratio of up to 1,391-fold.
“Micro-CT and confocal microscopy were game-changers,” says Traverso. “Micro-CT helped us map the internal structure of both the natural remora discs and our synthetic replicas, while confocal imaging allowed us to visualize how water was displaced within the compartments – confirming the vacuum-seal mechanism in action.” Kang also highlights that particle image velocimetry allowed his team to model fluid dynamics during adhesion, “something rarely captured so precisely in soft tissue adhesion studies,” he says.
One “aha” moment for the team was realizing just how much of the remora’s success lies in its ability to unfurl its disc and create multidirectional suction. “That insight completely reshaped our design approach,” says Kang. “Another big surprise was how small variations in lamella angle – something we observed in remora species that attach to soft hosts – made a massive difference in adhesion strength when we mimicked them in the lab.”
The team are now exploring a range of use cases for the technology, such as biosensing, drug delivery, or medical diagnostics.
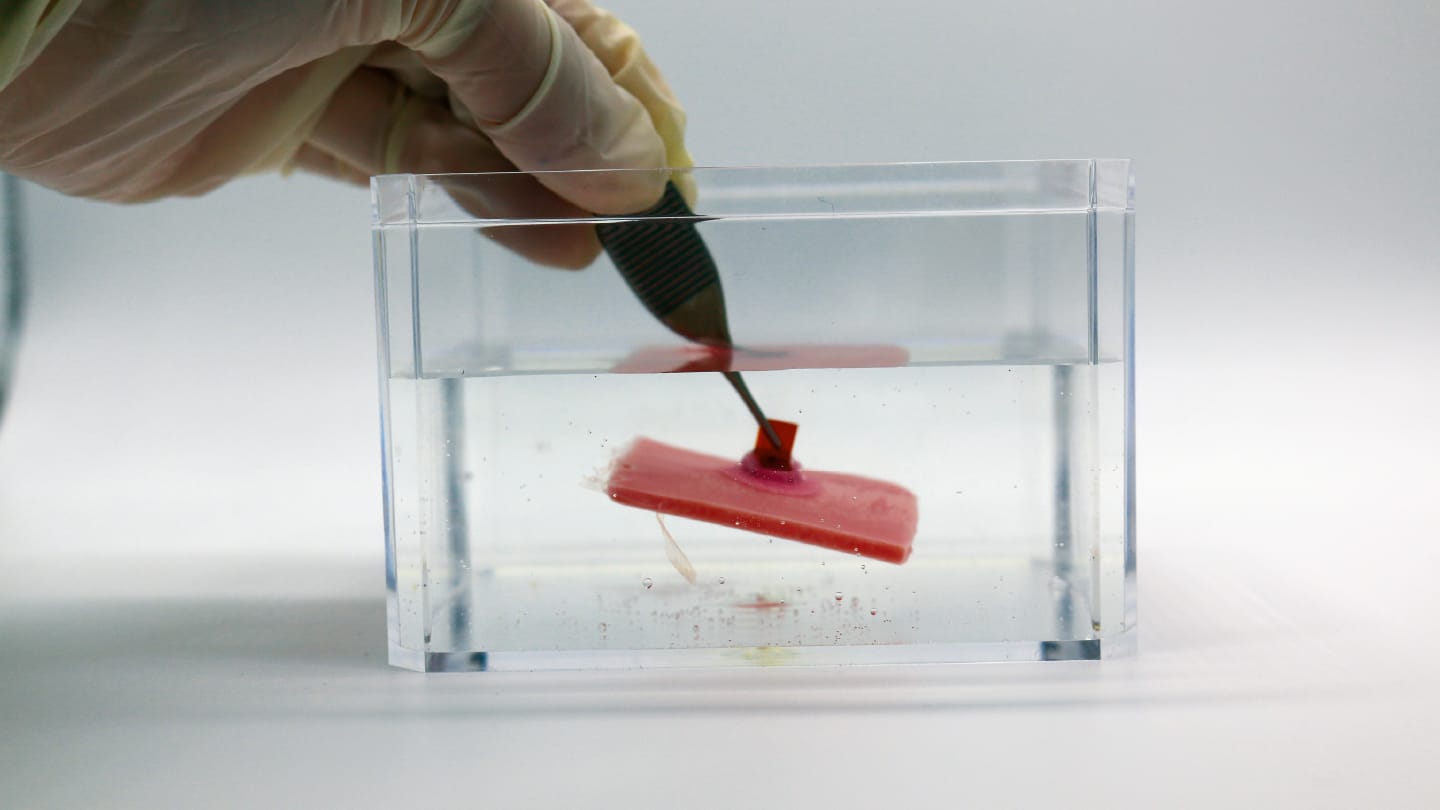
“GERD monitoring and mucosal mRNA delivery are probably closest to clinical translation,” says Traverso. “With GERD, we’ve already shown that MUSAS can safely and non-invasively hold a pH sensor in the esophagus. And for mRNA delivery, our system achieved high local expression in pigs – a huge step forward for non-invasive mucosal vaccination or gene therapy. The platform’s passive, motor-free nature makes it especially attractive for outpatient or at-home use.”
The researchers will now turn their attention to the tricky translational task of navigating regulatory pathways and scaling manufacturing while preserving function. “Fortunately, MUSAS is constructed from FDA-approved materials and fits within a size-000 capsule – so from a format perspective, it’s already optimized for oral delivery,” says Kang. “The challenge will be tailoring the design for specific applications – like tuning retention time or integrating electronics – without compromising safety or scalability.
“What excites us is that we're just scratching the surface of biomimicry. Systems like MUSAS show that you don’t always need complex electronics or chemical adhesives. Sometimes, borrowing a page from a fish’s playbook and leaning into mechanical simplicity can lead to transformative solutions for healthcare and beyond.”




